Embedment of Quantum Dots and Biomolecules in a Dipeptide Hydrogel Formed In Situ Using Microfluidics
- PMID: 33283395
- PMCID: PMC7986802
- DOI: 10.1002/anie.202015340
Embedment of Quantum Dots and Biomolecules in a Dipeptide Hydrogel Formed In Situ Using Microfluidics
Abstract
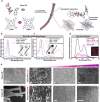
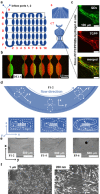
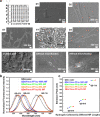
As low-molecular-weight hydrogelators, dipeptide hydrogel materials are suited for embedding multiple organic molecules and inorganic nanoparticles. Herein, a simple but precisely controllable method is presented that enables the fabrication of dipeptide-based hydrogels by supramolecular assembly inside microfluidic channels. Water-soluble quantum dots (QDs) as well as premixed porphyrins and a dipeptide in dimethyl sulfoxide (DMSO) were injected into a Y-shaped microfluidic junction. At the DMSO/water interface, the confined fabrication of a dipeptide-based hydrogel was initiated. Thereafter, the as-formed hydrogel flowed along a meandering microchannel in a continuous fashion, gradually completing gelation and QD entrapment. In contrast to hydrogelation in conventional test tubes, microfluidically controlled hydrogelation led to a tailored dipeptide hydrogel regarding material morphology and nanoparticle distribution.
Keywords: continuous-flow microfluidics; dipeptides; microchannel-confined assembly; nanostructures; supramolecular assembly.
© 2020 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

