Safety and immunogenicity of inactivated poliovirus vaccine schedules for the post-eradication era: a randomised open-label, multicentre, phase 3, non-inferiority trial
- PMID: 33284114
- PMCID: PMC7992032
- DOI: 10.1016/S1473-3099(20)30555-7
Safety and immunogenicity of inactivated poliovirus vaccine schedules for the post-eradication era: a randomised open-label, multicentre, phase 3, non-inferiority trial
Abstract
Background: Following the global eradication of wild poliovirus, countries using live attenuated oral poliovirus vaccines will transition to exclusive use of inactivated poliovirus vaccine (IPV) or fractional doses of IPV (f-IPV; a f-IPV dose is one-fifth of a normal IPV dose), but IPV supply and cost constraints will necessitate dose-sparing strategies. We compared immunisation schedules of f-IPV and IPV to inform the choice of optimal post-eradication schedule.
Methods: This randomised open-label, multicentre, phase 3, non-inferiority trial was done at two centres in Panama and one in the Dominican Republic. Eligible participants were healthy 6-week-old infants with no signs of febrile illness or known allergy to vaccine components. Infants were randomly assigned (1:1:1:1, 1:1:1:2, 2:1:1:1), using computer-generated blocks of four or five until the groups were full, to one of four groups and received: two doses of intradermal f-IPV (administered at 14 and 36 weeks; two f-IPV group); or three doses of intradermal f-IPV (administered at 10, 14, and 36 weeks; three f-IPV group); or two doses of intramuscular IPV (administered at 14 and 36 weeks; two IPV group); or three doses of intramuscular IPV (administered at 10, 14, and 36 weeks; three IPV group). The primary outcome was seroconversion rates based on neutralising antibodies for poliovirus type 1 and type 2 at baseline and at 40 weeks (4 weeks after the second or third vaccinations) in the per-protocol population to allow non-inferiority and eventually superiority comparisons between vaccines and regimens. Three co-primary outcomes concerning poliovirus types 1 and 2 were to determine if seroconversion rates at 40 weeks of age after a two-dose regimen (administered at weeks 14 and 36) of intradermally administered f-IPV were non-inferior to a corresponding two-dose regimen of intramuscular IPV; if seroconversion rates at 40 weeks of age after a two-dose IPV regimen (weeks 14 and 36) were non-inferior to those after a three-dose IPV regimen (weeks 10, 14, and 36); and if seroconversion rates after a two-dose f-IPV regimen (weeks 14 and 36) were non-inferior to those after a three-dose f-IPV regimen (weeks 10, 14, and 36). The non-inferiority boundary was set at -10% for the lower bound of the two-sided 95% CI for the seroconversion rate difference.. Safety was assessed as serious adverse events and important medical events. This study is registered on ClinicalTrials.gov, NCT03239496.
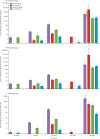
Findings: From Oct 23, 2017, to Nov 13, 2018, we enrolled 773 infants (372 [48%] girls) in Panama and the Dominican Republic (two f-IPV group n=217, three f-IPV group n=178, two IPV group n=178, and three IPV group n=200). 686 infants received all scheduled vaccine doses and were included in the per-protocol analysis. We observed non-inferiority for poliovirus type 1 seroconversion rate at 40 weeks for the two f-IPV dose schedule (95·9% [95% CI 92·0-98·2]) versus the two IPV dose schedule (98·7% [95·4-99·8]), and for the three f-IPV dose schedule (98·8% [95·6-99·8]) versus the three IPV dose schedule (100% [97·9-100]). Similarly, poliovirus type 2 seroconversion rate at 40 weeks for the two f-IPV dose schedule (97·9% [94·8-99·4]) versus the two IPV dose schedule (99·4% [96·4-100]), and for the three f-IPV dose schedule (100% [97·7-100]) versus the three IPV dose schedule (100% [97·9-100]) were non-inferior. Seroconversion rate for the two f-IPV regimen was statistically superior 4 weeks after the last vaccine dose in the 14 and 36 week schedule (95·9% [92·0-98·2]) compared with the 10 and 14 week schedule (83·2% [76·5-88·6]; p=0·0062) for poliovirus type 1. Statistical superiority of the 14 and 36 week schedule was also found for poliovirus type 2 (14 and 36 week schedule 97·9% [94·8-99·4] vs 10 and 14 week schedule 83·9% [77·2-89·2]; p=0·0062), and poliovirus type 3 (14 and 36 week schedule 84·5% [78·7-89·3] vs 10 and 14 week schedule 73·3% [65·8-79·9]; p=0·0062). For IPV, a two dose regimen administered at 14 and 36 weeks (99·4% [96·4-100]) was superior a 10 and 14 week schedule (88·9% [83·4-93·1]; p<0·0001) for poliovirus type 2, but not for type 1 (14 and 36 week schedule 98·7% [95·4-99·8] vs 10 and 14 week schedule 95·6% [91·4-98·1]), or type 3 (14 and 36 week schedule 97·4% [93·5-99·3] vs 10 and 14 week schedule 93·9% [89·3-96·9]). There were no related serious adverse events or important medical events reported in any group showing safety was unaffected by administration route or schedule.
Interpretation: Our observations suggest that adequate immunity against poliovirus type 1 and type 2 is provided by two doses of either IPV or f-IPV at 14 and 36 weeks of age, and broad immunity is provided with three doses of f-IPV, enabling substantial savings in cost and supply. These novel clinical data will inform global polio immunisation policy for the post-eradication era.
Funding: Bill & Melinda Gates Foundation.
Copyright © 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures

Similar articles
-
Inactivated poliovirus vaccine given alone or in a sequential schedule with bivalent oral poliovirus vaccine in Chilean infants: a randomised, controlled, open-label, phase 4, non-inferiority study.Lancet Infect Dis. 2015 Nov;15(11):1273-82. doi: 10.1016/S1473-3099(15)00219-4. Epub 2015 Aug 26. Lancet Infect Dis. 2015. PMID: 26318714 Clinical Trial.
-
Humoral and intestinal immunity induced by new schedules of bivalent oral poliovirus vaccine and one or two doses of inactivated poliovirus vaccine in Latin American infants: an open-label randomised controlled trial.Lancet. 2016 Jul 9;388(10040):158-69. doi: 10.1016/S0140-6736(16)00703-0. Epub 2016 May 19. Lancet. 2016. PMID: 27212429 Clinical Trial.
-
Immunogenicity and safety of three aluminium hydroxide adjuvanted vaccines with reduced doses of inactivated polio vaccine (IPV-Al) compared with standard IPV in young infants in the Dominican Republic: a phase 2, non-inferiority, observer-blinded, randomised, and controlled dose investigation trial.Lancet Infect Dis. 2017 Jul;17(7):745-753. doi: 10.1016/S1473-3099(17)30177-9. Epub 2017 Apr 25. Lancet Infect Dis. 2017. PMID: 28454674 Free PMC article. Clinical Trial.
-
Equivalent schedules of intradermal fractional dose versus intramuscular full dose of inactivated polio vaccine for prevention of poliomyelitis.Cochrane Database Syst Rev. 2019 Dec 19;12(12):CD011780. doi: 10.1002/14651858.CD011780.pub2. Cochrane Database Syst Rev. 2019. PMID: 31858595 Free PMC article.
-
Sequential inactivated (IPV) and live oral (OPV) poliovirus vaccines for preventing poliomyelitis.Cochrane Database Syst Rev. 2019 Dec 5;12(12):CD011260. doi: 10.1002/14651858.CD011260.pub2. Cochrane Database Syst Rev. 2019. PMID: 31801180 Free PMC article.
Cited by
-
Inactivated Poliovirus Vaccine Closing the Type 2 Immunity Gap in Vietnam.J Pediatric Infect Dis Soc. 2022 Sep 29;11(9):413-416. doi: 10.1093/jpids/piac046. J Pediatric Infect Dis Soc. 2022. PMID: 35801634 Free PMC article.
-
Assessing and Mitigating Local Vulnerabilities to Completeness of Global Polio Eradication.J Pediatric Infect Dis Soc. 2022 Jan 27;11(1):3-4. doi: 10.1093/jpids/piab102. J Pediatric Infect Dis Soc. 2022. PMID: 34730816 Free PMC article. No abstract available.
-
Genetic stabilization of attenuated oral vaccines against poliovirus types 1 and 3.Nature. 2023 Jul;619(7968):135-142. doi: 10.1038/s41586-023-06212-3. Epub 2023 Jun 14. Nature. 2023. PMID: 37316671 Free PMC article.
-
Assessing the immunogenicity of three different inactivated polio vaccine schedules for use after oral polio vaccine cessation, an open label, phase IV, randomized controlled trial.Vaccine. 2021 Sep 24;39(40):5814-5821. doi: 10.1016/j.vaccine.2021.08.065. Epub 2021 Sep 2. Vaccine. 2021. PMID: 34481702 Free PMC article. Clinical Trial.
-
Maintaining the Region of the Americas free of polio: best practices for incident management support teams.Rev Panam Salud Publica. 2024 Apr 1;48:e23. doi: 10.26633/RPSP.2024.23. eCollection 2024. Rev Panam Salud Publica. 2024. PMID: 38562959 Free PMC article.
References
-
- Global Polio Eradication Initiative Polio endgame strategy 2019-2023: eradication, integration, certification and containment. 2019. http://polioeradication.org/wp-content/uploads/2019/06/english-polio-end...
-
- Sabin AB. Oral poliovirus vaccine: history of its development and use and current challenge to eliminate poliomyelitis from the world. J Infect Dis. 1985;151:420–423. - PubMed
-
- Global Polio Eradication Initiative Wild poliovirus list: List of wild poliovirus by country and year. 2010. http://polioeradication.org/polio-today/polio-now/wild-poliovirus-list/
-
- WHO Meeting of the Strategic Advisory Group of Experts on immunization, October 2015—conclusions and recommendations. Wkly Epidemiol Rec. 2015;50:681–700. - PubMed
-
- Bandyopadhyay AS, Garon J, Seib K, Orenstein WA. Polio vaccination: past, present and future. Future Microbiol. 2015;10:791–808. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

