Preclinical comparison of four [18F, natGa]rhPSMA-7 isomers: influence of the stereoconfiguration on pharmacokinetics
- PMID: 33284394
- PMCID: PMC7721954
- DOI: 10.1186/s13550-020-00740-z
Preclinical comparison of four [18F, natGa]rhPSMA-7 isomers: influence of the stereoconfiguration on pharmacokinetics
Abstract
Introduction: Radiohybrid (rh) ligands, a novel class of prostate-specific membrane antigen (PSMA)-targeted radiopharmaceuticals, can be labeled either with [18F]fluorine via isotopic exchange or with radiometals (such as [68Ga]Gallium, [177Lu]Lutetium, [225Ac]Actinium). Among these, [18F, natGa]rhPSMA-7 has recently entered clinical assessment.
Aim: Since [18F, natGa]rhPSMA-7 is composed of four stereoisomers ([18F, natGa]rhPSMA-7.1, -7.2, -7.3 and -7.4), we initiated a preclinical selection process to identify the isomer with the most favorable pharmacokinetics for further clinical investigation.
Methods: A synthetic protocol for enantiopure [19F, natGa]rhPSMA-7 isomers has been developed. The comparative evaluation of the four isomers comprised human serum albumin binding, lipophilicity, IC50, internalization and classical biodistribution studies and competition experiments in LNCaP tumor-bearing CB-17 SCID mice. In addition, a radio high-performance liquid chromatography-based method was developed allowing quantitative, intraindividual comparison of [18F, natGa]rhPSMA-7.1 to -7.4 in LNCaP tumor-bearing mice.
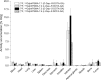
Results: Cell studies revealed high PSMA affinity and internalization for [18/19F, natGa]rhPSMA-7.2, -7.3 and -7.4, whereas [18/19F, natGa]rhPSMA-7.1 showed approximately twofold lower values. Although the biodistribution profile obtained was typical of PSMA inhibitors, it did not allow for selection of a lead candidate for clinical studies. Thus, an intraindividual comparison of all four isomers in LNCaP tumor-bearing mice was carried out by injection of a diastereomeric mixture, followed by analysis of the differential uptake and excretion pattern of each isomer. Based on its high tumor accumulation and low uptake in blood, liver and kidneys, [18F, natGa]rhPSMA-7.3 was identified as the preferred isomer and transferred into clinical studies.
Conclusion: [18F, natGa]rhPSMA-7.3 has been selected as a lead compound for clinical development of a [18F]rhPSMA-based candidate. The intraindividual differential uptake and excretion analysis in vivo allowed for an accurate comparison and assessment of radiopharmaceuticals.
Keywords: Fluorine-18; PSMA; Prostate cancer; Radiohybrid.
Conflict of interest statement
HJW and AW are listed as inventors on the patent application for rhPSMA. HJW is founder, shareholder and scientific advisor of Scintomics GmbH, Fuerstenfeldbruck, Germany. No other potential conflicts of interest relevant to this article exist.
Figures




References
-
- Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395(10231):1208–1216. doi: 10.1016/S0140-6736(20)30314-7. - DOI - PubMed
-
- Afshar-Oromieh A, Malcher A, Eder M, Eisenhut M, Linhart HG, Hadaschik BA, et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging. 2013;40(4):486–495. doi: 10.1007/s00259-012-2298-2. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

