Hydroxychloroquine as Postexposure Prophylaxis to Prevent Severe Acute Respiratory Syndrome Coronavirus 2 Infection : A Randomized Trial
- PMID: 33284679
- PMCID: PMC7732017
- DOI: 10.7326/M20-6519
Hydroxychloroquine as Postexposure Prophylaxis to Prevent Severe Acute Respiratory Syndrome Coronavirus 2 Infection : A Randomized Trial
Erratum in
-
Correction: Hydroxychloroquine as Postexposure Prophylaxis to Prevent Severe Acute Respiratory Syndrome Coronavirus 2 Infection.Ann Intern Med. 2021 Mar;174(3):435. doi: 10.7326/L21-0009. Ann Intern Med. 2021. PMID: 33721531 No abstract available.
Abstract
Background: Effective prevention against coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is currently limited to nonpharmaceutical strategies. Laboratory and observational data suggested that hydroxychloroquine had biological activity against SARS-CoV-2, potentially permitting its use for prevention.
Objective: To test hydroxychloroquine as postexposure prophylaxis for SARS-CoV-2 infection.
Design: Household-randomized, double-blind, controlled trial of hydroxychloroquine postexposure prophylaxis. (ClinicalTrials.gov: NCT04328961).
Setting: National U.S. multicenter study.
Participants: Close contacts recently exposed (<96 hours) to persons with diagnosed SARS-CoV-2 infection.
Intervention: Hydroxychloroquine (400 mg/d for 3 days followed by 200 mg/d for 11 days) or ascorbic acid (500 mg/d followed by 250 mg/d) as a placebo-equivalent control.
Measurements: Participants self-collected mid-turbinate swabs daily (days 1 to 14) for SARS-CoV-2 polymerase chain reaction (PCR) testing. The primary outcome was PCR-confirmed incident SARS-CoV-2 infection among persons who were SARS-CoV-2 negative at enrollment.
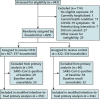
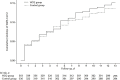
Results: Between March and August 2020, 671 households were randomly assigned: 337 (407 participants) to the hydroxychloroquine group and 334 (422 participants) to the control group. Retention at day 14 was 91%, and 10 724 of 11 606 (92%) expected swabs were tested. Among the 689 (89%) participants who were SARS-CoV-2 negative at baseline, there was no difference between the hydroxychloroquine and control groups in SARS-CoV-2 acquisition by day 14 (53 versus 45 events; adjusted hazard ratio, 1.10 [95% CI, 0.73 to 1.66]; P > 0.20). The frequency of participants experiencing adverse events was higher in the hydroxychloroquine group than the control group (66 [16.2%] versus 46 [10.9%], respectively; P = 0.026).
Limitation: The delay between exposure, and then baseline testing and the first dose of hydroxychloroquine or ascorbic acid, was a median of 2 days.
Conclusion: This rigorous randomized controlled trial among persons with recent exposure excluded a clinically meaningful effect of hydroxychloroquine as postexposure prophylaxis to prevent SARS-CoV-2 infection.
Primary funding source: Bill & Melinda Gates Foundation.
Conflict of interest statement
Figures
References
-
- World Health Organization. Transmission of SARS-CoV-2: implications for infection prevention precautions. 9 July 2020. Accessed at www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-imp... on 24 August 2020.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
