Antenatal corticosteroid administration and early school age child development: A regression discontinuity study in British Columbia, Canada
- PMID: 33284805
- PMCID: PMC7721186
- DOI: 10.1371/journal.pmed.1003435
Antenatal corticosteroid administration and early school age child development: A regression discontinuity study in British Columbia, Canada
Abstract
Background: There are growing concerns that antenatal corticosteroid administration may harm children's neurodevelopment. We investigated the safety of antenatal corticosteroid administration practices for children's overall developmental health (skills and behaviors) at early school age.
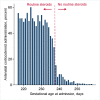
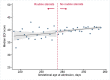
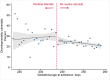
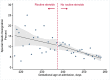
Methods and findings: We linked population health and education databases from British Columbia (BC), Canada to identify a cohort of births admitted to hospital between 31 weeks, 0 days gestation (31+0 weeks), and 36+6 weeks, 2000 to 2013, with routine early school age child development testing. We used a regression discontinuity design to compare outcomes of infants admitted just before and just after the clinical threshold for corticosteroid administration of 34+0 weeks. We estimated the median difference in the overall Early Development Instrument (EDI) score and EDI subdomain scores, as well as risk differences (RDs) for special needs designation and developmental vulnerability (<10th percentile on 2 or more subdomains). The cohort included 5,562 births admitted between 31+0 and 36+6 weeks, with a median EDI score of 40/50. We found no evidence that antenatal corticosteroid administration practices were linked with altered child development at early school age: median EDI score difference of -0.5 [95% CI: -2.2 to 1.7] (p = 0.65), RD per 100 births for special needs designation -0.5 [-4.2 to 3.1] (p = 0.96) and for developmental vulnerability of 3.9 [95% CI:-2.2 to 10.0] (p = 0.24). A limitation of our study is that the regression discontinuity design estimates the effect of antenatal corticosteroid administration at the gestational age of the discontinuity, 34 + 0 weeks, so our results may become less generalisable as gestational age moves further away from this point.
Conclusions: Our study did not find that that antenatal corticosteroid administration practices were associated with child development at early school age. Our findings may be useful for supporting clinical counseling about antenatal corticosteroids administration at late preterm gestation, when the balance of harms and benefits is less clear.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Royal College of Obstetricians and Gynaecologists. Antenatal Corticosteroids to Reduce Neonatal Morbidity and Mortality. London, UK, 2010.
-
- National Institutes of Health. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consens Statement. 1994; 12(2): 1–24. - PubMed
-
- National Institute for Health and Care Excellence. Preterm Labour and Birth. NICE Guideline. 2019;25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

