A large meta-analysis establishes the role of MRD negativity in long-term survival outcomes in patients with multiple myeloma
- PMID: 33284948
- PMCID: PMC7724898
- DOI: 10.1182/bloodadvances.2020002827
A large meta-analysis establishes the role of MRD negativity in long-term survival outcomes in patients with multiple myeloma
Abstract
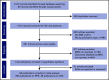
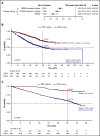
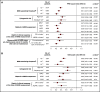
The prognostic value of minimal residual disease (MRD) for progression-free survival (PFS) and overall survival (OS) was evaluated in a large cohort of patients with multiple myeloma (MM) using a systematic literature review and meta-analysis. Medline and EMBASE databases were searched for articles published up to 8 June 2019, with no date limit on the indexed database. Clinical end points stratified by MRD status (positive or negative) were extracted, including hazard ratios (HRs) on PFS and OS, P values, and confidence intervals (CIs). HRs were estimated based on reconstructed patient-level data from published Kaplan-Meier curves. Forty-four eligible studies with PFS data from 8098 patients, and 23 studies with OS data from 4297 patients were identified to assess the association between MRD status and survival outcomes. Compared with MRD positivity, achieving MRD negativity improved PFS (HR, 0.33; 95% CI, 0.29-0.37; P < .001) and OS (HR, 0.45; 95% CI, 0.39-0.51; P < .001). MRD negativity was associated with significantly improved survival outcomes regardless of disease setting (newly diagnosed or relapsed/refractory MM), MRD sensitivity thresholds, cytogenetic risk, method of MRD assessment, depth of clinical response at the time of MRD measurement, and MRD assessment premaintenance and 12 months after start of maintenance therapy. The strong prognostic value of MRD negativity and its association with favorable outcomes in various disease and treatment settings sets the stage to adopt MRD as a treatment end point, including development of therapeutic strategies. This large meta-analysis confirms the utility of MRD as a relevant surrogate for PFS and OS in MM.
Conflict of interest statement
Conflict--interest disclosure: N.C.M. has received consulting fees from AbbVie, Adaptive, Amgen, Celgene, Janssen, Legend, Oncopep, and Takeda. H.A.-L. has received consulting fees, research funding, and travel and lecture fees from AbbVie, Amgen, BMS/Celgene, Janssen, Sanofi, and Takeda. K.C.A. has received consulting fees from Bristol-Myers Squibb, Celgene, Gilead, Janssen, Precision Biosciences, Sanofi-Aventis, Takeda, and Tolero. P.N. has received consulting fees, honoraria, and research funding from Amgen, Celgene, and Janssen. B.P. has received consulting fees from Celgene, Janssen, and Sanofi; was on the advisory boards and received honoraria for lectures from Adaptive, Amgen, Bristol-Myers Squibb, Celgene, Janssen, Merck, Novartis, Roche, Sanofi, and Takeda; and received unrestricted grants from Celgene, EngMab, Sanofi, and Takeda. M.D. has received consulting fees, honoraria, and research funding from Amgen, Celgene, Janssen, and Takeda and participated in advisory boards for Sanofi-Aventis. M.K., M.H., and B.H. are employees of Ingress-Health. A.L., J.H., J.U., J.V., and S.C. are employees of Janssen. N.B. has received consulting fees and honoraria from AbbVie, Celgene, Janssen, Karyopharm, and Takeda. M.S. declares no competing financial interests.
Figures



References
-
- Gay F, Cerrato C, Petrucci MT, et al. Efficacy of carfilzomib lenalidomide dexamethasone (KRd) with or without transplantation in newly diagnosed myeloma according to risk status: results from the FORTE trial. J Clin Oncol. 2019;37(15 suppl):8002.
-
- Yoroidaka T, Takamatsu H, Yamashita T, et al. Minimal residual disease assessment using Euroflow-NGF in patients with multiple myeloma treated with a combination of carfilzomib, lenalidomide, and dexamethasone (KRD). Blood. 2019;134(suppl 1):3130.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

