Polyphosphazene immunoadjuvants: Historical perspective and recent advances
- PMID: 33285104
- PMCID: PMC7904599
- DOI: 10.1016/j.jconrel.2020.12.001
Polyphosphazene immunoadjuvants: Historical perspective and recent advances
Abstract
The development of successful vaccines has been increasingly reliant on the use of immunoadjuvants - additives, which can enhance and modulate immune responses to vaccine antigens. Immunoadjuvants of the polyphosphazene family encompass synthetic biodegradable macromolecules, which attain in vivo activity via antigen delivery and immunostimulation mechanisms. Over the last decades, the technology has witnessed evolvement of next generation members, expansion to include various antigens and routes of administration, and progression to clinical phase. This was accompanied by gaining important insights into the mechanism of action and the development of a novel class of virus-mimicking nano-assemblies for antigen delivery. The present review evaluates in vitro and in vivo data generated to date in the context of latest advances in understanding the primary function and biophysical behavior of these macromolecules. It also provides an overview of relevant synthetic and characterization methods, macromolecular biodegradation pathways, and polyphosphazene-based multi-component, nanoparticulate, and microfabricated formulations.
Keywords: Antigens; Biodegradable polymers; Immunoadjuvants; Nanocomplexes; Polyphosphazenes; Proteins; Resiquimod; TLR agonists; Vaccine delivery.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Conflict of Interest
A.K.A. declares no conflict of interest. For a list of entities with which R.L. is involved, compensated or uncompensated, see
Figures

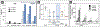



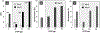

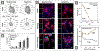


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

