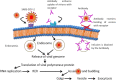
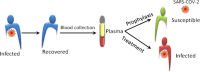Emergence of ancient convalescent plasma (CP) therapy: To manage COVID-19 pandemic
- PMID: 33285298
- PMCID: PMC7718590
- DOI: 10.1016/j.tracli.2020.11.004
Emergence of ancient convalescent plasma (CP) therapy: To manage COVID-19 pandemic
Abstract
Since December 2019, the human populations of the 195 global countries continue experiencing grave health and life threats due to the current COVID-19 pandemic. As a result of the novelty of the pathogen, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), at present there is lack of preventive as well as therapeutic options for treating and managing the infection. The use of ancient immunotherapeutic technique - the convalescent plasma (CP) therapy, may act as an immediate and available option to control the COVID-19 pandemic. This review provides a concept and understanding on the CP therapy, its potential to control SARS-CoV-2 pandemic. The CP therapy might act as an immediate saviour for society from the virus. Although the CP therapy has exert affirmative result against COVID-19 it has not been recommended for long time use in COVID-19 and this review gives support for its possible application.
Keywords: Antibody-dependent enhancement (ADE); Convalescent plasma (CP) therapy; Coronavirus disease 2019 (COVID-19); Passive immune therapy; Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Copyright © 2020. Published by Elsevier Masson SAS.
Figures
Similar articles
-
Convalescent plasma - Is it useful for treating SARS Co-V2 infection?Indian J Med Microbiol. 2020 Jul-Dec;38(3 & 4):252-260. doi: 10.4103/ijmm.IJMM_20_358. Indian J Med Microbiol. 2020. PMID: 33154232 Free PMC article. Review.
-
Treatment for emerging viruses: Convalescent plasma and COVID-19.Transfus Apher Sci. 2020 Jun;59(3):102790. doi: 10.1016/j.transci.2020.102790. Epub 2020 Apr 20. Transfus Apher Sci. 2020. PMID: 32345485 Free PMC article. Review.
-
Convalescent plasma for pediatric patients with SARS-CoV-2-associated acute respiratory distress syndrome.Pediatr Blood Cancer. 2020 Nov;67(11):e28693. doi: 10.1002/pbc.28693. Epub 2020 Sep 4. Pediatr Blood Cancer. 2020. PMID: 32885904 Free PMC article.
-
Convalescent Plasma Therapy for COVID-19: Lessons from SARS-CoV, MERS-CoV, and H1N1 Infection.Acta Med Indones. 2021 Jan;53(1):86-95. Acta Med Indones. 2021. PMID: 33818411 Review.
-
Production of anti-SARS-CoV-2 hyperimmune globulin from convalescent plasma.Transfusion. 2021 Jun;61(6):1705-1709. doi: 10.1111/trf.16378. Epub 2021 Mar 22. Transfusion. 2021. PMID: 33715160 Free PMC article.
Cited by
-
A comprehensive review on pharmacologic agents, immunotherapies and supportive therapeutics for COVID-19.Narra J. 2022 Dec;2(3):e92. doi: 10.52225/narra.v2i3.92. Epub 2022 Dec 8. Narra J. 2022. PMID: 38449903 Free PMC article. Review.
-
Antibody-dependent enhancement of coronaviruses.Int J Biol Sci. 2025 Feb 3;21(4):1686-1704. doi: 10.7150/ijbs.96112. eCollection 2025. Int J Biol Sci. 2025. PMID: 39990674 Free PMC article. Review.
-
Stem cell therapies and benefaction of somatic cell nuclear transfer cloning in COVID-19 era.Stem Cell Res Ther. 2021 May 12;12(1):283. doi: 10.1186/s13287-021-02334-5. Stem Cell Res Ther. 2021. PMID: 33980321 Free PMC article. Review.
-
Theoretical Explanation for the Rarity of Antibody-Dependent Enhancement of Infection (ADE) in COVID-19.Int J Mol Sci. 2022 Sep 26;23(19):11364. doi: 10.3390/ijms231911364. Int J Mol Sci. 2022. PMID: 36232664 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous