Manual lymphatic drainage for lymphedema in patients after breast cancer surgery: A systematic review and meta-analysis of randomized controlled trials
- PMID: 33285693
- PMCID: PMC7717855
- DOI: 10.1097/MD.0000000000023192
Manual lymphatic drainage for lymphedema in patients after breast cancer surgery: A systematic review and meta-analysis of randomized controlled trials
Abstract
Background: Studies have shown that manual lymphatic drainage (MLD) has a beneficial effect on lymphedema related to breast cancer surgery. However, whether MLD reduces the risk of lymphedema is still debated. The purpose of this systematic review and meta-analysis was to summarize the current evidence to assess the effectiveness of MLD in preventing and treating lymphedema in patients after breast cancer surgery.
Methods: From inception to May 2019, PubMed, EMBASE, and Cochrane Library databases were systematically searched without language restriction. We included randomized controlled trials (RCTs) that compared the treatment and prevention effect of MLD with a control group on lymphedema in breast cancer patients. A random-effects model was used for all analyses.
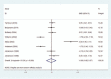
Results: A total of 17 RCTs involving 1911 patients were included. A meta-analysis of 8 RCTs, including 338 patients, revealed that MLD did not significantly reduce lymphedema compared with the control group (standardized mean difference (SMD): -0.09, 95% confidence interval (CI): [-0.85 to 0.67]). Subgroup analysis was basically consistent with the main analysis according to the research region, the publication year, the sample size, the type of surgery, the statistical analysis method, the mean age, and the intervention time. However, we found that MLD could significantly reduce lymphedema in patients under the age of 60 years (SMD: -1.77, 95% CI: [-2.23 to -1.31]) and an intervention time of 1 month (SMD: -1.77, 95% CI: [-2.23 to -1.30]). Meanwhile, 4 RCTs including, 1364 patients, revealed that MLD could not significantly prevent the risk of lymphedema (risk ratio (RR): 0.61, 95% CI: [0.29-1.26]) for patients having breast cancer surgery.
Conclusions: Overall, this meta-analysis of 12 RCTs showed that MLD cannot significantly reduce or prevent lymphedema in patients after breast cancer surgery. However, well-designed RCTs with a larger sample size are required, especially in patients under the age of 60 years or an intervention time of 1 month.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures





References
-
- Zou L, Liu F-H, Shen P-P, et al. The incidence and risk factors of related lymphedema for breast cancer survivors post-operation: a 2-year follow-up prospective cohort study. Breast Cancer (Tokyo, Japan) 2018;25:309–14. - PubMed
-
- Ribeiro Pereira ACP, Koifman RJ, Bergmann A, et al. Incidence and risk factors of lymphedema after breast cancer treatment: 10 years of follow-up. Breast (Edinburgh, Scotland) 2017;36:67–73. - PubMed
-
- Kasseroller RE. The Vodder school: the Vodder method. Cancer 1998;83: Suppl.: 2840–2. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

