Anti-Influenza Strategies Based on Nanoparticle Applications
- PMID: 33287259
- PMCID: PMC7761763
- DOI: 10.3390/pathogens9121020
Anti-Influenza Strategies Based on Nanoparticle Applications
Abstract
Influenza virus has the potential for being one of the deadliest viruses, as we know from the pandemic's history. The influenza virus, with a constantly mutating genome, is becoming resistant to existing antiviral drugs and vaccines. For that reason, there is an urgent need for developing new therapeutics and therapies. Despite the fact that a new generation of universal vaccines or anti-influenza drugs are being developed, the perfect remedy has still not been found. In this review, various strategies for using nanoparticles (NPs) to defeat influenza virus infections are presented. Several categories of NP applications are highlighted: NPs as immuno-inducing vaccines, NPs used in gene silencing approaches, bare NPs influencing influenza virus life cycle and the use of NPs for drug delivery. This rapidly growing field of anti-influenza methods based on nanotechnology is very promising. Although profound research must be conducted to fully understand and control the potential side effects of the new generation of antivirals, the presented and discussed studies show that nanotechnology methods can effectively induce the immune responses or inhibit influenza virus activity both in vitro and in vivo. Moreover, with its variety of modification possibilities, nanotechnology has great potential for applications and may be helpful not only in anti-influenza but also in the general antiviral approaches.
Keywords: antisense strategies; delivery; influenza; influenza vaccine; influenza virus; inhibition; nanoparticles; nanotechnology; resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

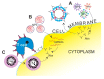
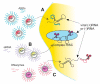

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

