Autonomous and Assisted Control for Synthetic Microbiology
- PMID: 33287299
- PMCID: PMC7731081
- DOI: 10.3390/ijms21239223
Autonomous and Assisted Control for Synthetic Microbiology
Abstract
The control of microbes and microbial consortia to achieve specific functions requires synthetic circuits that can reliably cope with internal and external perturbations. Circuits that naturally evolved to regulate biological functions are frequently robust to alterations in their parameters. As the complexity of synthetic circuits increases, synthetic biologists need to implement such robust control "by design". This is especially true for intercellular signaling circuits for synthetic consortia, where robustness is highly desirable, but its mechanisms remain unclear. Cybergenetics, the interface between synthetic biology and control theory, offers two approaches to this challenge: external (computer-aided) and internal (autonomous) control. Here, we review natural and synthetic microbial systems with robustness, and outline experimental approaches to implement such robust control in microbial consortia through population-level cybergenetics. We propose that harnessing natural intercellular circuit topologies with robust evolved functions can help to achieve similar robust control in synthetic intercellular circuits. A "hybrid biology" approach, where robust synthetic microbes interact with natural consortia and-additionally-with external computers, could become a useful tool for health and environmental applications.
Keywords: control; cybergenetics; microbial consortia; relative sensing; robustness; synthetic biology.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
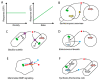
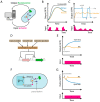
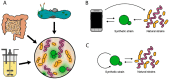
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

