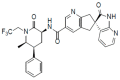
Pharmacokinetics, Pharmacodynamics and Drug-Drug Interactions of New Anti-Migraine Drugs-Lasmiditan, Gepants, and Calcitonin-Gene-Related Peptide (CGRP) Receptor Monoclonal Antibodies
- PMID: 33287305
- PMCID: PMC7761673
- DOI: 10.3390/pharmaceutics12121180
Pharmacokinetics, Pharmacodynamics and Drug-Drug Interactions of New Anti-Migraine Drugs-Lasmiditan, Gepants, and Calcitonin-Gene-Related Peptide (CGRP) Receptor Monoclonal Antibodies
Abstract
In the last few years, there have been significant advances in migraine management and prevention. Lasmiditan, ubrogepant, rimegepant and monoclonal antibodies (erenumab, fremanezumab, galcanezumab, and eptinezumab) are new drugs that were launched on the US pharmaceutical market; some of them also in Europe. This publication reviews the available worldwide references on the safety of these anti-migraine drugs with a focus on the possible drug-drug (DDI) or drug-food interactions. As is known, bioavailability of a drug and, hence, its pharmacological efficacy depend on its pharmacokinetics and pharmacodynamics, which may be altered by drug interactions. This paper discusses the interactions of gepants and lasmiditan with, i.a., serotonergic drugs, CYP3A4 inhibitors, and inducers or breast cancer resistant protein (BCRP) and P-glycoprotein (P-gp) inhibitors. In the case of monoclonal antibodies, the issue of pharmacodynamic interactions related to the modulation of the immune system functions was addressed. It also focuses on the effect of monoclonal antibodies on expression of class Fc gamma receptors (FcγR).
Keywords: drug–drug interactions; gepants; lasmiditan; migraine; monoclonal antibodies.
Conflict of interest statement
The author declares no conflict of interest.
Figures
References
-
- Szkutnik-Fiedler D. Current pharmacotherapy of migraine. Headache and COVID-19. Farm Wsp. 2020;13:76–83. (In Polish)
-
- Greb E. Headache May Predict Clinical Evolution of COVID-19. [(accessed on 25 June 2020)]; Available online: https://www.medscape.com/viewarticle/932637#vp_2.
-
- American Headache Society (AHS) Annual Meeting 2020: Presented. [(accessed on 30 June 2020)];2020 Jun 13; Available online: https://americanheadachesociety.org/events/virtual-annual-scientific-mee...
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous