Focused Ultrasound-Induced Cavitation Sensitizes Cancer Cells to Radiation Therapy and Hyperthermia
- PMID: 33287379
- PMCID: PMC7761886
- DOI: 10.3390/cells9122595
Focused Ultrasound-Induced Cavitation Sensitizes Cancer Cells to Radiation Therapy and Hyperthermia
Abstract
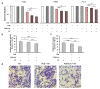
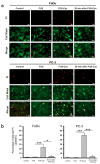
Focused ultrasound (FUS) has become an important non-invasive therapy for solid tumor ablation via thermal effects. The cavitation effect induced by FUS is thereby avoided but applied for lithotripsy, support drug delivery and the induction of blood vessel destruction for cancer therapy. In this study, head and neck cancer (FaDu), glioblastoma (T98G), and prostate cancer (PC-3) cells were exposed to FUS by using an in vitro FUS system followed by single-dose X-ray radiation therapy (RT) or water bath hyperthermia (HT). Sensitization effects of short FUS shots with cavitation (FUS-Cav) or without cavitation (FUS) to RT or HT (45 °C, 30 min) were evaluated. FUS-Cav significantly increases the sensitivity of cancer cells to RT and HT by reducing long-term clonogenic survival, short-term cell metabolic activity, cell invasion, and induction of sonoporation. Our results demonstrated that short FUS treatment with cavitation has good potential to sensitize cancer cells to RT and HT non-invasively.
Keywords: cavitation; focused ultrasound; hyperthermia; radiation therapy; sensitization.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
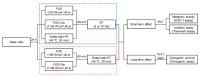
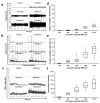
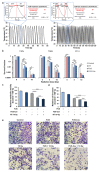
References
-
- Siedek F., Yeo S.Y., Heijman E., Grinstein O., Bratke G., Heneweer C., Puesken M., Persigehl T., Maintz D., Grüll H. Magnetic Resonance-Guided High-Intensity Focused Ultrasound (MR-HIFU): Technical Background and Overview of Current Clinical Applications (Part 1) RöFo—Fortschr. Geb. Röntgenstrahlen Bildgeb. Verfahr. 2019;191:522–530. doi: 10.1055/a-0817-5645. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

