The Arabidopsis Mitochondrial Glutaredoxin GRXS15 Provides [2Fe-2S] Clusters for ISCA-Mediated [4Fe-4S] Cluster Maturation
- PMID: 33287436
- PMCID: PMC7730481
- DOI: 10.3390/ijms21239237
The Arabidopsis Mitochondrial Glutaredoxin GRXS15 Provides [2Fe-2S] Clusters for ISCA-Mediated [4Fe-4S] Cluster Maturation
Abstract
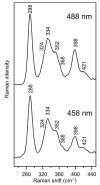
Iron-sulfur (Fe-S) proteins are crucial for many cellular functions, particularly those involving electron transfer and metabolic reactions. An essential monothiol glutaredoxin GRXS15 plays a key role in the maturation of plant mitochondrial Fe-S proteins. However, its specific molecular function is not clear, and may be different from that of the better characterized yeast and human orthologs, based on known properties. Hence, we report here a detailed characterization of the interactions between Arabidopsis thaliana GRXS15 and ISCA proteins using both in vivo and in vitro approaches. Yeast two-hybrid and bimolecular fluorescence complementation experiments demonstrated that GRXS15 interacts with each of the three plant mitochondrial ISCA1a/1b/2 proteins. UV-visible absorption/CD and resonance Raman spectroscopy demonstrated that coexpression of ISCA1a and ISCA2 resulted in samples with one [2Fe-2S]2+ cluster per ISCA1a/2 heterodimer, but cluster reconstitution using as-purified [2Fe-2S]-ISCA1a/2 resulted in a [4Fe-4S]2+ cluster-bound ISCA1a/2 heterodimer. Cluster transfer reactions monitored by UV-visible absorption and CD spectroscopy demonstrated that [2Fe-2S]-GRXS15 mediates [2Fe-2S]2+ cluster assembly on mitochondrial ferredoxin and [4Fe-4S]2+ cluster assembly on the ISCA1a/2 heterodimer in the presence of excess glutathione. This suggests that ISCA1a/2 is an assembler of [4Fe-4S]2+ clusters, via two-electron reductive coupling of two [2Fe-2S]2+ clusters. Overall, the results provide new insights into the roles of GRXS15 and ISCA1a/2 in effecting [2Fe-2S]2+ to [4Fe-4S]2+ cluster conversions for the maturation of client [4Fe-4S] cluster-containing proteins in plants.
Keywords: Arabidopsis thaliana; ISCA proteins; Raman spectroscopy; circular dichroism; glutaredoxin; iron-sulfur cluster trafficking; iron-sulfur protein; mitochondria; protein-protein interaction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Dong G., Witcher S., Outten F.W., Pilon M. The Suf system in Archaea, Bacteria, and Eukaryotic organelles. In: Johnson M.K., Scott R.A., editors. Encyclopedia of Inorganic and Bioinorganic Chemistry: Metalloprotein Site Assembly. John Wiley and Sons; Chichester, UK: 2017. pp. 37–52.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

