Mitochondria and Calcium Homeostasis: Cisd2 as a Big Player in Cardiac Ageing
- PMID: 33287440
- PMCID: PMC7731030
- DOI: 10.3390/ijms21239238
Mitochondria and Calcium Homeostasis: Cisd2 as a Big Player in Cardiac Ageing
Abstract
The ageing of human populations has become a problem throughout the world. In this context, increasing the healthy lifespan of individuals has become an important target for medical research and governments. Cardiac disease remains the leading cause of morbidity and mortality in ageing populations and results in significant increases in healthcare costs. Although clinical and basic research have revealed many novel insights into the pathways that drive heart failure, the molecular mechanisms underlying cardiac ageing and age-related cardiac dysfunction are still not fully understood. In this review we summarize the most updated publications and discuss the central components that drive cardiac ageing. The following characters of mitochondria-related dysfunction have been identified during cardiac ageing: (a) disruption of the integrity of mitochondria-associated membrane (MAM) contact sites; (b) dysregulation of energy metabolism and dynamic flexibility; (c) dyshomeostasis of Ca2+ control; (d) disturbance to mitochondria-lysosomal crosstalk. Furthermore, Cisd2, a pro-longevity gene, is known to be mainly located in the endoplasmic reticulum (ER), mitochondria, and MAM. The expression level of Cisd2 decreases during cardiac ageing. Remarkably, a high level of Cisd2 delays cardiac ageing and ameliorates age-related cardiac dysfunction; this occurs by maintaining correct regulation of energy metabolism and allowing dynamic control of metabolic flexibility. Together, our previous studies and new evidence provided here highlight Cisd2 as a novel target for developing therapies to promote healthy ageing.
Keywords: Cisd2; calcium homeostasis; cardiac ageing; energy flexibility; mitochondria; mitochondria-associated membranes (MAMs).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


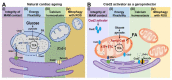
References
-
- Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Delling F.N., et al. Heart Disease and Stroke Statistics-2020 Update: A Report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. - DOI - PubMed
-
- Lee K.-S., Huh S., Lee S., Wu Z., Kim A.-K., Kang H.-Y., Lu B. Altered ER-mitochondria contact impacts mitochondria calcium homeostasis and contributes to neurodegeneration in vivo in disease models. Proc. Natl. Acad. Sci. USA. 2018;115:E8844–E8853. doi: 10.1073/pnas.1721136115. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MOST107-2314-B-182A-160-MY3/Ministry of Science and Technology, Taiwan
- MOST109-2320-B-182A-020/Ministry of Science and Technology, Taiwan
- MOST 109-2320-B-010-043/Ministry of Science and Technology, Taiwan
- MOST 109-2634-F-010-003/Ministry of Science and Technology, Taiwan
- CMRPD5J0021/Chang Gung Memorial Hospital
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

