Prior exposure of Arabidopsis seedlings to mechanical stress heightens jasmonic acid-mediated defense against necrotrophic pathogens
- PMID: 33287718
- PMCID: PMC7720613
- DOI: 10.1186/s12870-020-02759-9
Prior exposure of Arabidopsis seedlings to mechanical stress heightens jasmonic acid-mediated defense against necrotrophic pathogens
Abstract
Background: Prolonged mechanical stress (MS) causes thigmomorphogenesis, a stress acclimation response associated with increased disease resistance. What remains unclear is if; 1) plants pre-exposed to a short period of repetitive MS can prime defence responses upon subsequent challenge with necrotrophic pathogens, 2) MS mediates plant immunity via jasmonic acid (JA) signalling, and 3) a short period of repetitive MS can cause long-term changes in gene expression resembling a stress-induced memory. To address these points, 10-days old juvenile Arabidopsis seedlings were mechanically stressed for 7-days using a soft brush and subsequently challenged with the necrotrophic pathogens, Alternaria brassicicola, and Botrytis cinerea. Here we assessed how MS impacted structural cell wall appositions, disease symptoms and altered gene expression in response to infection.
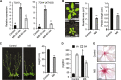
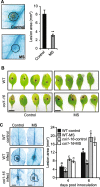
Results: The MS-treated plants exhibited enhanced cell wall appositions and jasmonic acid (JA) accumulation that correlated with a reduction in disease progression compared to unstressed plants. The expression of genes involved in JA signalling, callose deposition, peroxidase and phytoalexin biosynthesis and reactive oxygen species detoxification were hyper-induced 4-days post-infection in MS-treated plants. The loss-of-function in JA signalling mediated by the JA-insensitive coronatine-insensitive 1 (coi1) mutant impaired the hyper-induction of defense gene expression and promoted pathogen proliferation in MS-treated plants subject to infection. The basal expression level of PATHOGENESIS-RELATED GENE 1 and PLANT DEFENSIN 1.2 defense marker genes were constitutively upregulated in rosette leaves for 5-days post-MS, as well as in naïve cauline leaves that differentiated from the inflorescence meristem well after ceasing MS.
Conclusion: This study reveals that exposure of juvenile Arabidopsis plants to a short repetitive period of MS can alter gene expression and prime plant resistance upon subsequent challenge with necrotrophic pathogens via the JA-mediated COI1 signalling pathway. MS may facilitate a stress-induced memory to modulate the plant's response to future stress encounters. These data advance our understanding of how MS primes plant immunity against necrotrophic pathogens and how that could be utilised in sustainable agricultural practices.
Keywords: Alternaria brassicicola; Botrytis cinerea; Jasmonic acid; Mechanical stress; Necrotrophic pathogen; Stress priming.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Short-Term Exposure to Nitrogen Dioxide Provides Basal Pathogen Resistance.Plant Physiol. 2018 Sep;178(1):468-487. doi: 10.1104/pp.18.00704. Epub 2018 Aug 3. Plant Physiol. 2018. PMID: 30076223 Free PMC article.
-
Arabidopsis Elongator subunit 2 positively contributes to resistance to the necrotrophic fungal pathogens Botrytis cinerea and Alternaria brassicicola.Plant J. 2015 Sep;83(6):1019-33. doi: 10.1111/tpj.12946. Epub 2015 Aug 17. Plant J. 2015. PMID: 26216741
-
Priming for JA-dependent defenses using hexanoic acid is an effective mechanism to protect Arabidopsis against B. cinerea.J Plant Physiol. 2011 Mar 1;168(4):359-66. doi: 10.1016/j.jplph.2010.07.028. Epub 2010 Oct 14. J Plant Physiol. 2011. PMID: 20950893
-
Jasmonic Acid as a Mediator in Plant Response to Necrotrophic Fungi.Cells. 2023 Mar 27;12(7):1027. doi: 10.3390/cells12071027. Cells. 2023. PMID: 37048100 Free PMC article. Review.
-
Baking bad: plants in a toasty world with necrotrophs.New Phytol. 2024 Sep;243(6):2066-2072. doi: 10.1111/nph.19980. Epub 2024 Jul 22. New Phytol. 2024. PMID: 39039780 Review.
Cited by
-
Bacillus velezensis GH1-13 enhances drought tolerance in rice by reducing the accumulation of reactive oxygen species.Front Plant Sci. 2024 Sep 25;15:1432494. doi: 10.3389/fpls.2024.1432494. eCollection 2024. Front Plant Sci. 2024. PMID: 39391772 Free PMC article.
-
A Novel Guanine Elicitor Stimulates Immunity in Arabidopsis and Rice by Ethylene and Jasmonic Acid Signaling Pathways.Front Plant Sci. 2022 Feb 17;13:841228. doi: 10.3389/fpls.2022.841228. eCollection 2022. Front Plant Sci. 2022. PMID: 35251109 Free PMC article.
-
Pathogen-derived mechanical cues potentiate the spatio-temporal implementation of plant defense.BMC Biol. 2022 Dec 27;20(1):292. doi: 10.1186/s12915-022-01495-w. BMC Biol. 2022. PMID: 36575418 Free PMC article.
-
Vegetal memory through the lens of transcriptomic changes - recent progress and future practical prospects for exploiting plant transcriptional memory.Plant Signal Behav. 2024 Dec 31;19(1):2383515. doi: 10.1080/15592324.2024.2383515. Epub 2024 Jul 30. Plant Signal Behav. 2024. PMID: 39077764 Free PMC article. Review.
-
Function and Mechanism of Jasmonic Acid in Plant Responses to Abiotic and Biotic Stresses.Int J Mol Sci. 2021 Aug 9;22(16):8568. doi: 10.3390/ijms22168568. Int J Mol Sci. 2021. PMID: 34445272 Free PMC article. Review.
References
-
- Braam J. In touch: plant responses to mechanical stimuli. New Phytol. 2005;165(2):373-89. - PubMed
-
- Telewski FW, Jaffe MJ. Thigmomorphogenesis - changes in the morphology and chemical-composition induced by mechanical perturbation in 6-month-old Pinus-Taeda seedlings. Can J Forest Res Rev Canadienne De Recherche Forestiere. 1981;11(2):380–387.
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

