Efficacy of 104-week Telbivudine-based optimization strategy in patients with HBeAg-negative chronic hepatitis B virus infections
- PMID: 33287722
- PMCID: PMC7720458
- DOI: 10.1186/s12879-020-05642-y
Efficacy of 104-week Telbivudine-based optimization strategy in patients with HBeAg-negative chronic hepatitis B virus infections
Abstract
Background: Evaluate the safety and efficacy of 104-week regimen of Telbivudine(LdT)-based optimization strategy for Chinese patients who have chronic hepatits B(CHB) with HBeAg-negative.
Methods: This multi-center, open-label, prospective study enrolled 108 HBeAg-negative CHB patients who received LdT (600 mg/day) for 24 weeks, Adefovir (ADV) was added if HBV DNA remained detectable at week 24, otherwise LdT was maintained to use until 104 weeks. HBV DNA, alanine amino transferase (ALT), hepatitis B surface antigen(HBsAg), creatinine kinase(CK), and estimated glomerular filtration rate (eGFR) were measured, safety was assessed.
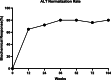
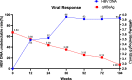
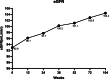
Results: Eighty-eight patients (81%) had HBV-DNA undetectable at 24 weeks and maintained to receive LdT monotherapy until 104 weeks, whereas the other 20 patients had HBV-DNA detectable and ADV was used in combination. For all patients, 72% of patients reached ALT normalization at 24 weeks, which increased to 80% at 52 weeks and 104 weeks, respectively.. 81% of total patients had undetectable HBV-DNA at 24 weeks, 92% at 52 weeks, and 94% at 104 weeks. The HBsAg titre declined steadily from baseline to 104 weeks (3.62 vs. 2.98 log10 IU/mL, p < 0.05), and the eGFR increased steadily from baseline to 104 weeks (92.9 vs. 104.4 mL/min/1.73 m2, p < 0.05). Although 79 patients (73%) had at least one time of elevated CK, most of these patients had CK elevated in Grade 1/2.
Conclusions: LdT was well tolerated and effective, and 94% of patients achieved virological suppression after 104 weeks.
Trial registration: This study was registered in clinicaltrials.gov on January 31, 2012 and the ID No. was NCT01521975 .
Keywords: Chronic hepatitis B; HBeAg; Telbivudine; eGFR.
Conflict of interest statement
All authors declare that they have no competing interest.
Figures
References
-
- World Health Organization. March 2015 [cited 2015 May 5].
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous