Randomized controlled multicentre study of albumin replacement therapy in septic shock (ARISS): protocol for a randomized controlled trial
- PMID: 33287911
- PMCID: PMC7720035
- DOI: 10.1186/s13063-020-04921-y
Randomized controlled multicentre study of albumin replacement therapy in septic shock (ARISS): protocol for a randomized controlled trial
Abstract
Background: Albumin is a key regulator of fluid distribution within the extracellular space and has several properties beyond its oncotic activity. The accumulating evidence suggests that supplementation of albumin may provide survival advantages only when the insult is severe as in patients with septic shock.
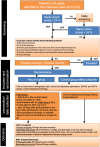
Methods/design: The randomized controlled multicentre study of albumin replacement therapy in septic shock (ARISS) investigates whether the replacement with albumin and the maintenance of its serum levels of at least 30 g/l for 28 days improve survival in patients with septic shock compared to resuscitation and volume maintenance without albumin. Adult patients (≥ 18 years) with septic shock are randomly assigned within a maximum of 24 h after the onset of septic shock after obtaining informed consents to treatment or control groups. Patients assigned to the treatment group receive a 60-g loading dose of human albumin 20% over 2-3 h. Serum albumin levels are maintained at least at 30 g/l in the ICU for a maximum of 28 days following randomization using 40-80 g human albumin 20% infusion. The control group is treated according to the usual practice with crystalloids as the first choice for the resuscitation and maintenance phase of septic shock. The primary endpoint is 90 days mortality and secondary endpoints include 28-day, 60-day, ICU, and in-hospital mortality, organ dysfunction/failure, total amount of fluid administration and total fluid balance in the ICU, and lengths of ICU and hospital stay. In total, 1412 patients need to be analysed, 706 per group. For the sample size estimation, a 15% reduction in 90-day mortality is assumed, i.e. an absolute reduction of 7.5% points to 42.5% (relative risk 1.18). Assuming a dropout rate of 15%, a total of 1662 patients need to be allocated.
Discussion: The results of the clinical trial may influence the treatment of patients with septic shock. The expected improvement in patient survival may result in a reduction in the resources currently used in the treatment of these patients and in the socioeconomic burden of this disease.
Trial registration: ClinicalTrials.gov NCT03869385 . Registration on 18 July 2019. Protocol version: Final 3.0.
Keywords: Albumin; Fluid resuscitation; Septic shock.
Conflict of interest statement
S. Kluge received research support by Ambu, E.T.View Ltd., Fisher & Paykel, Pfizer, and Xenios. He also received lecture honorarium from Arjo Huntleigh, Astellas, Astra, Basilea, Bard, Baxter, Biotest, CSL Behring, Cytosorbents, Fresenius, Gilead, MSD, Orion, Pfizer, Philips, Sedana, Sorin, Xenios, and Zoll. He received consultant honorarium from AMOMED, Astellas, Baxter, Bayer, Fresenius, Gilead, MSD, Pfizer, and Xenios. Y Sakr received consultation and lecture honorarium from Grifols S.A., in addition to the support supplied for the current trial. All other others declare that they do not have conflict of interest in relation to the subject of the current manuscript.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

