Humanized C3 Mouse: A Novel Accelerated Model of C3 Glomerulopathy
- PMID: 33288630
- PMCID: PMC7894673
- DOI: 10.1681/ASN.2020050698
Humanized C3 Mouse: A Novel Accelerated Model of C3 Glomerulopathy
Abstract
Background: C3 glomerulopathy (C3G) is characterized by the alternative-pathway (AP) hyperactivation induced by nephritic factors or complement gene mutations. Mice deficient in complement factor H (CFH) are a classic C3G model, with kidney disease that requires several months to progress to renal failure. Novel C3G models can further contribute to understanding the mechanism behind this disease and developing therapeutic approaches.
Methods: A novel, rapidly progressing, severe, murine model of C3G was developed by replacing the mouse C3 gene with the human C3 homolog using VelociGene technology. Functional, histologic, molecular, and pharmacologic assays characterize the presentation of renal disease and enable useful pharmacologic interventions in the humanized C3 (C3hu/hu) mice.
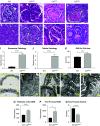
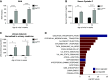
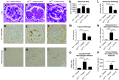
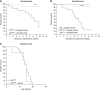
Results: The C3hu/hu mice exhibit increased morbidity early in life and die by about 5-6 months of age. The C3hu/hu mice display elevated biomarkers of kidney dysfunction, glomerulosclerosis, C3/C5b-9 deposition, and reduced circulating C3 compared with wild-type mice. Administration of a C5-blocking mAb improved survival rate and offered functional and histopathologic benefits. Blockade of AP activation by anti-C3b or CFB mAbs also extended survival and preserved kidney function.
Conclusions: The C3hu/hu mice are a useful model for C3G because they share many pathologic features consistent with the human disease. The C3G phenotype in C3hu/hu mice may originate from a dysregulated interaction of human C3 protein with multiple mouse complement proteins, leading to unregulated C3 activation via AP. The accelerated disease course in C3hu/hu mice may further enable preclinical studies to assess and validate new therapeutics for C3G.
Keywords: C2 KO; C3 glomerulopathy; C3b; C5; CFH mAbs; accelerated kidney disease; humanized C3 mice; liver phenotype; mouse model.
Copyright © 2021 by the American Society of Nephrology.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

