Absence of Lenacapavir (GS-6207) Phenotypic Resistance in HIV Gag Cleavage Site Mutants and in Isolates with Resistance to Existing Drug Classes
- PMID: 33288639
- PMCID: PMC8092519
- DOI: 10.1128/AAC.02057-20
Absence of Lenacapavir (GS-6207) Phenotypic Resistance in HIV Gag Cleavage Site Mutants and in Isolates with Resistance to Existing Drug Classes
Abstract
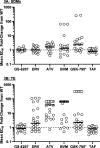
Lenacapavir (LEN; GS-6207) is a potent first-in-class inhibitor of HIV-1 capsid with long-acting properties and the potential for subcutaneous dosing every 3 months or longer. In the clinic, a single subcutaneous LEN injection (20 mg to 750 mg) in people with HIV (PWH) induced a strong antiviral response, with a >2.3 mean log10 decrease in HIV-1 RNA at day 10. HIV-1 Gag mutations near protease (PR) cleavage sites have emerged with the use of protease inhibitors (PIs). Here, we have characterized the activity of LEN in mutants with Gag cleavage site mutations (GCSMs) and mutants resistant to other drug classes. HIV mutations were inserted into the pXXLAI clone, and the resulting mutants (n = 70) were evaluated using a 5-day antiviral assay. LEN EC50 fold change versus the wild type ranged from 0.4 to 1.9 in these mutants, similar to that for the control drug. In contrast, reduced susceptibility to PIs and maturation inhibitors (MIs) was observed. Testing of isolates with resistance against the 4 main classes of drugs (n = 40) indicated wild-type susceptibility to LEN (fold change ranging from 0.3 to 1.1), while reduced susceptibility was observed for control drugs. HIV GCSMs did not impact the activity of LEN, while some conferred resistance to MIs and PIs. Similarly, LEN activity was not affected by naturally occurring variations in HIV Gag, in contrast to the reduced susceptibility observed for MIs. Finally, the activity of LEN was not affected by the presence of resistance mutations to the 4 main antiretroviral (ARV) drug classes. These data support the evaluation of LEN in PWH with multiclass resistance.
Keywords: GS-6207; HIV-1; antiretroviral resistance; capsid inhibitor; cross-resistance.
Copyright © 2021 American Society for Microbiology.
Figures


References
-
- Yant SR, Mulato A, Hansen D, Tse WC, Niedziela-Majka A, Zhang JR, Stepan GJ, Jin D, Wong MH, Perreira JM, Singer E, Papalia GA, Hu EY, Zheng J, Lu B, Schroeder SD, Chou K, Ahmadyar S, Liclican A, Yu H, Novikov N, Paoli E, Gonik D, Ram RR, Hung M, McDougall WM, Brass AL, Sundquist WI, Cihlar T, Link JO. 2019. A highly potent long-acting small-molecule HIV-1 capsid inhibitor with efficacy in a humanized mouse model. Nat Med 25:1377–1384. doi: 10.1038/s41591-019-0560-x. - DOI - PMC - PubMed
-
- Link JO, Rhee MS, Tse WC, Zheng J, Somoza JR, Rowe W, Begley R, Chiu A, Mulato A, Hansen D, Singer E, Tsai LK, Bam RA, Chou CH, Canales E, Brizgys G, Zhang JR, Li J, Graupe M, Morganelli P, Liu Q, Wu Q, Halcomb RL, Saito RD, Schroeder SD, Lazerwith SE, Bondy S, Jin D, Hung M, Novikov N, Liu X, Villasenor AG, Cannizzaro CE, Hu EY, Anderson RL, Appleby TC, Lu B, Mwangi J, Liclican A, Niedziela-Majka A, Papalia GA, Wong MH, Leavitt SA, Xu Y, Koditek D, Stepan GJ, Yu H, Pagratis N, Clancy S, Ahmadyar S, et al. 2020. Clinical targeting of HIV capsid protein with a long-acting small molecule. Nature 584:614–618. doi: 10.1038/s41586-020-2443-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

