Single-molecule fluorescence-based approach reveals novel mechanistic insights into human small heat shock protein chaperone function
- PMID: 33288678
- PMCID: PMC7921601
- DOI: 10.1074/jbc.RA120.015419
Single-molecule fluorescence-based approach reveals novel mechanistic insights into human small heat shock protein chaperone function
Abstract
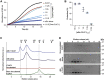
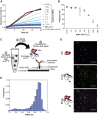
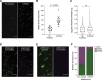
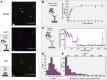
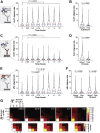
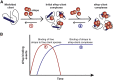
Small heat shock proteins (sHsps) are a family of ubiquitous intracellular molecular chaperones; some sHsp family members are upregulated under stress conditions and play a vital role in protein homeostasis (proteostasis). It is commonly accepted that these chaperones work by trapping misfolded proteins to prevent their aggregation; however, fundamental questions regarding the molecular mechanism by which sHsps interact with misfolded proteins remain unanswered. The dynamic and polydisperse nature of sHsp oligomers has made studying them challenging using traditional biochemical approaches. Therefore, we have utilized a single-molecule fluorescence-based approach to observe the chaperone action of human alphaB-crystallin (αBc, HSPB5). Using this approach we have, for the first time, determined the stoichiometries of complexes formed between αBc and a model client protein, chloride intracellular channel 1. By examining the dispersity and stoichiometries of these complexes over time, and in response to different concentrations of αBc, we have uncovered unique and important insights into a two-step mechanism by which αBc interacts with misfolded client proteins to prevent their aggregation.
Keywords: alphaB-crystallin; chloride intracellular channel 1; mass photometry; molecular chaperone; oligomers; protein aggregation; protein complexes; total internal reflection fluorescence microscopy.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures

References
-
- Jakob U., Gaestel M., Engel K., Buchner J. Small heat shock proteins are molecular chaperones. J. Biol. Chem. 1993;268:1517–1520. - PubMed
-
- de Jong W.W., Leunissen J.A., Voorter C. Evolution of the alpha-crystallin/small heat-shock protein family. Mol. Biol. Evol. 1993;10:103–126. - PubMed
-
- Clark J.I., Muchowski P.J. Small heat-shock proteins and their potential role in human disease. Curr. Opin. Struct. Biol. 2000;10:52–59. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

