The mechanoresponse of bone is closely related to the osteocyte lacunocanalicular network architecture
- PMID: 33288694
- PMCID: PMC7768754
- DOI: 10.1073/pnas.2011504117
The mechanoresponse of bone is closely related to the osteocyte lacunocanalicular network architecture
Abstract
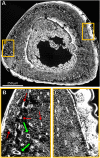
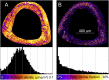
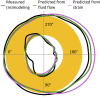
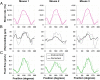
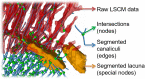
Organisms rely on mechanosensing mechanisms to adapt to changes in their mechanical environment. Fluid-filled network structures not only ensure efficient transport but can also be employed for mechanosensation. The lacunocanalicular network (LCN) is a fluid-filled network structure, which pervades our bones and accommodates a cell network of osteocytes. For the mechanism of mechanosensation, it was hypothesized that load-induced fluid flow results in forces that can be sensed by the cells. We use a controlled in vivo loading experiment on murine tibiae to test this hypothesis, whereby the mechanoresponse was quantified experimentally by in vivo micro-computed tomography (µCT) in terms of formed and resorbed bone volume. By imaging the LCN using confocal microscopy in bone volumes covering the entire cross-section of mouse tibiae and by calculating the fluid flow in the three-dimensional (3D) network, we could perform a direct comparison between predictions based on fluid flow velocity and the experimentally measured mechanoresponse. While local strain distributions estimated by finite-element analysis incorrectly predicts preferred bone formation on the periosteal surface, we demonstrate that additional consideration of the LCN architecture not only corrects this erroneous bias in the prediction but also explains observed differences in the mechanosensitivity between the three investigated mice. We also identified the presence of vascular channels as an important mechanism to locally reduce fluid flow. Flow velocities increased for a convergent network structure where all of the flow is channeled into fewer canaliculi. We conclude that, besides mechanical loading, LCN architecture should be considered as a key determinant of bone adaptation.
Keywords: bone adaptation; fluid flow; in vivo µCT; lacunocanalicular network; mechanobiology.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Verbruggen S. W., Mechanobiology in Health and Disease (Academic Press, 2018).
-
- le Noble F., et al. , Control of arterial branching morphogenesis in embryogenesis: Go with the flow. Cardiovasc. Res. 65, 619–628 (2005). - PubMed
-
- Klein-Nulend J., Bakker A. D., Bacabac R. G., Vatsa A., Weinbaum S., Mechanosensation and transduction in osteocytes. Bone 54, 182–190 (2013). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

