DIA-based systems biology approach unveils E3 ubiquitin ligase-dependent responses to a metabolic shift
- PMID: 33288721
- PMCID: PMC7768684
- DOI: 10.1073/pnas.2020197117
DIA-based systems biology approach unveils E3 ubiquitin ligase-dependent responses to a metabolic shift
Abstract
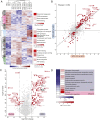
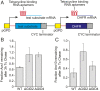
The yeast Saccharomyces cerevisiae is a powerful model system for systems-wide biology screens and large-scale proteomics methods. Nearly complete proteomics coverage has been achieved owing to advances in mass spectrometry. However, it remains challenging to scale this technology for rapid and high-throughput analysis of the yeast proteome to investigate biological pathways on a global scale. Here we describe a systems biology workflow employing plate-based sample preparation and rapid, single-run, data-independent mass spectrometry analysis (DIA). Our approach is straightforward, easy to implement, and enables quantitative profiling and comparisons of hundreds of nearly complete yeast proteomes in only a few days. We evaluate its capability by characterizing changes in the yeast proteome in response to environmental perturbations, identifying distinct responses to each of them and providing a comprehensive resource of these responses. Apart from rapidly recapitulating previously observed responses, we characterized carbon source-dependent regulation of the GID E3 ligase, an important regulator of cellular metabolism during the switch between gluconeogenic and glycolytic growth conditions. This unveiled regulatory targets of the GID ligase during a metabolic switch. Our comprehensive yeast system readout pinpointed effects of a single deletion or point mutation in the GID complex on the global proteome, allowing the identification and validation of targets of the GID E3 ligase. Moreover, this approach allowed the identification of targets from multiple cellular pathways that display distinct patterns of regulation. Although developed in yeast, rapid whole-proteome-based readouts can serve as comprehensive systems-level assays in all cellular systems.
Keywords: GID E3 ligase; mass spectrometry; proteomics; stress; yeast systems biology.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Berner N., Reutter K. R., Wolf D. H., Protein quality control of the endoplasmic reticulum and ubiquitin-proteasome-triggered degradation of aberrant proteins: Yeast pioneers the path. Annu. Rev. Biochem. 87, 751–782 (2018). - PubMed
-
- Hipp M. S., Park S. H., Hartl F. U., Proteostasis impairment in protein-misfolding and -aggregation diseases. Trends Cell Biol. 24, 506–514 (2014). - PubMed
-
- Steinmetz L. M., et al. , Systematic screen for human disease genes in yeast. Nat. Genet. 31, 400–404 (2002). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

