Structural insights into assembly and function of the RSC chromatin remodeling complex
- PMID: 33288924
- PMCID: PMC7855068
- DOI: 10.1038/s41594-020-00528-8
Structural insights into assembly and function of the RSC chromatin remodeling complex
Abstract
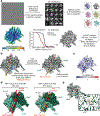
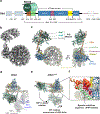
SWI/SNF chromatin remodelers modify the position and spacing of nucleosomes and, in humans, are linked to cancer. To provide insights into the assembly and regulation of this protein family, we focused on a subcomplex of the Saccharomyces cerevisiae RSC comprising its ATPase (Sth1), the essential actin-related proteins (ARPs) Arp7 and Arp9 and the ARP-binding protein Rtt102. Cryo-EM and biochemical analyses of this subcomplex shows that ARP binding induces a helical conformation in the helicase-SANT-associated (HSA) domain of Sth1. Surprisingly, the ARP module is rotated 120° relative to the full RSC about a pivot point previously identified as a regulatory hub in Sth1, suggesting that large conformational changes are part of Sth1 regulation and RSC assembly. We also show that a conserved interaction between Sth1 and the nucleosome acidic patch enhances remodeling. As some cancer-associated mutations dysregulate rather than inactivate SWI/SNF remodelers, our insights into RSC complex regulation advance a mechanistic understanding of chromatin remodeling in disease states.
Conflict of interest statement
Competing Interests
The authors declare no competing interests.
Figures





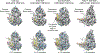









References
-
- Clapier CR & Cairns BR The Biology of Chromatin Remodeling Complexes. Annual Review of Biochemistry 78, 273–304 (2009). - PubMed
-
- Kwon H, Imbalzano AN, Khavari PA, Kingston RE & Green MR Nucleosome disruption and enhancement of activator binding by a human SW1/SNF complex. Nature 370, 477–481 (1994). - PubMed
-
- Cairns BR, Levinson RS, Yamamoto KR & Kornberg RD Essential role of Swp73p in the function of yeast Swi/Snf complex. Genes & Development 10, 2131–2144 (1996). - PubMed
Methods References
-
- Dyer PN et al. Reconstitution of Nucleosome Core Particles from Recombinant Histones and DNA in vol. 375 23–44 (2003). - PubMed
-
- Stark H Chapter Five GraFix: Stabilization of Fragile Macromolecular Complexes for Single Particle Cryo-EM. Methods in Enzymology 481, 109–126 (2010). - PubMed
-
- Kremer JR, Mastronarde DN & McIntosh JR Computer Visualization of Three-Dimensional Image Data Using IMOD. Journal of Structural Biology 116, 71–76 (1996). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

