Inhibition of fatty acid synthase with FT-4101 safely reduces hepatic de novo lipogenesis and steatosis in obese subjects with non-alcoholic fatty liver disease: Results from two early-phase randomized trials
- PMID: 33289350
- PMCID: PMC7898808
- DOI: 10.1111/dom.14272
Inhibition of fatty acid synthase with FT-4101 safely reduces hepatic de novo lipogenesis and steatosis in obese subjects with non-alcoholic fatty liver disease: Results from two early-phase randomized trials
Abstract
Aims: To assess the therapeutic potential of fatty acid synthase (FASN) inhibition with FT-4101, a potent, selective, orally bioavailable, small-molecule by (a) evaluating the dose-response of single FT-4101 doses (3, 6 and 9 mg) on hepatic de novo lipogenesis (DNL) in healthy participants (Study 1) and (b) demonstrating the safety, tolerability and efficacy on hepatic steatosis after 12 weeks of FT-4101 dosing in patients with non-alcoholic fatty liver disease (NAFLD; Study 2).
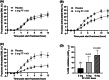
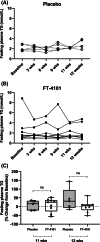
Materials and methods: In Study 1, three sequential cohorts of healthy men (n = 10/cohort) were randomized to receive a single dose of FT-4101 (n = 5/cohort) or placebo (n = 5/cohort) followed by crossover dosing after 7 days. Hepatic DNL was assessed during fructose stimulation from 13 C-acetate incorporation. In Study 2, men and women with NAFLD (n = 14) randomly received 12 weeks of intermittent once-daily dosing (four cycles of 2 weeks on-treatment, followed by 1 week off-treatment) of 3 mg FT-4101 (n = 9) or placebo (n = 5). Steady-state DNL based on deuterated water labelling, hepatic steatosis using magnetic resonance imaging-proton density fat fraction and sebum lipids and circulating biomarkers were assessed.
Results: Single and repeat dosing of FT-4101 were safe and well tolerated. Single FT-4101 doses inhibited hepatic DNL dose-dependently. Twelve weeks of 3 mg FT-4101 treatment improved hepatic steatosis and inhibited hepatic DNL. Decreases in sebum sapienate content with FT-4101 at week 11 were not significant compared to placebo and rebounded at week 12. Biomarkers of liver function, glucose and lipid metabolism were unchanged.
Conclusions: Inhibition of FASN with 3 mg FT-4101 safely reduces hepatic DNL and steatosis in NAFLD patients.
Keywords: drug development; drug mechanism; fatty liver disease; pharmacodynamics; phase I−II study; randomized trial.
© 2020 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
PS, EW, JB, MR, PK are employees and shareholders of Forma Therapeutics. WL and KD were employees of Forma Therapeutics at the time of contribution. CB receives consultancy fees from Forma Therapeutics and ProSciento, Inc. TER is an employee of Celerion. LM received consultancy fees from ProSciento, Inc. MH is an employee and shareholder of ProSciento, Inc. MKH and KL are employees of UCB. MKH receives consultancy fees from Forma Therapeutics. LJ is an employee and shareholder of Antaros Medical.
Figures



References
-
- Hellerstein MK. De novo lipogenesis in humans: metabolic and regulatory aspects. Eur J Clin Nutr. 1999;53(Suppl 1):S53‐S65. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

