Clinical utility of circulating tumor cells: an update
- PMID: 33289351
- PMCID: PMC8169442
- DOI: 10.1002/1878-0261.12869
Clinical utility of circulating tumor cells: an update
Abstract
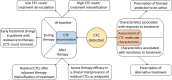
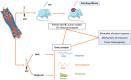
The prognostic role of circulating tumor cells (CTCs) has been clearly demonstrated in many types of cancer. However, their roles in diagnostic and treatment strategies remain to be defined. In this review, we present an overview of the current clinical validity of CTCs in nonmetastatic and metastatic cancer, and the main studies or concepts investigating the clinical utility of CTCs. In particular, we focus on breast, lung, colorectal, and prostate cancer. Two major topics concerning the clinical utility of CTC are discussed: treatment based on CTC count or CTC variations, and treatment based on the molecular characteristics of CTCs. Although some of these studies are inconclusive, many are still ongoing, and their results could help to define the role of CTCs in the management of cancers. A summary of published or ongoing phase II-III trials is also presented.
Keywords: CTC-derived xenografts; circulating tumor cells; clinical utility; clinical validity; liquid biopsy.
© 2020 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Conflict of interest statement
FCB and JYP report research grants from Menarini Silicon Biosystems and FCB reports travel support from Menarini Silicon Biosystems. Other authors declare no conflicts of interest.
Figures
References
-
- Ashworth TR (1869) A Case of cancer in which cells similar to those in the tumours were seen in the blood after death. Med J Aust 14, 146–147.
-
- Boya M, Chu C‐H, Liu R, Ozkaya‐Ahmadov T, Fatih Sarioglu A et al. (2020) Circulating tumor cell enrichment technologies. In Schaffner F, Merlin J‐L, von Bubnoff N (eds): Tumor Liquid Biopsies Vol. 215, pp. 25–55. Cham: Springer International Publishing. - PubMed
-
- Allard WJ (2004) Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 10, 6897–6904. - PubMed
-
- Kagan M, Howard D, Bendele T, Mayes J, Silvia J, Repollet M, Doyle J, Allard J, Tu N, Bui T et al. (2002) A sample preparation and analysis system for identification of circulating tumor cells. J Clin Ligand Assay 25, 104–110.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

