Sphingosine 1-phosphate Receptor Modulator Therapy for Multiple Sclerosis: Differential Downstream Receptor Signalling and Clinical Profile Effects
- PMID: 33289881
- PMCID: PMC7932974
- DOI: 10.1007/s40265-020-01431-8
Sphingosine 1-phosphate Receptor Modulator Therapy for Multiple Sclerosis: Differential Downstream Receptor Signalling and Clinical Profile Effects
Abstract
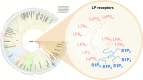
Lysophospholipids are a class of bioactive lipid molecules that produce their effects through various G protein-coupled receptors (GPCRs). Sphingosine 1-phosphate (S1P) is perhaps the most studied lysophospholipid and has a role in a wide range of physiological and pathophysiological events, via signalling through five distinct GPCR subtypes, S1PR1 to S1PR5. Previous and continuing investigation of the S1P pathway has led to the approval of three S1PR modulators, fingolimod, siponimod and ozanimod, as medicines for patients with multiple sclerosis (MS), as well as the identification of new S1PR modulators currently in clinical development, including ponesimod and etrasimod. S1PR modulators have complex effects on S1PRs, in some cases acting both as traditional agonists as well as agonists that produce functional antagonism. S1PR subtype specificity influences their downstream effects, including aspects of their benefit:risk profile. Some S1PR modulators are prodrugs, which require metabolic modification such as phosphorylation via sphingosine kinases, resulting in different pharmacokinetics and bioavailability, contrasting with others that are direct modulators of the receptors. The complex interplay of these characteristics dictates the clinical profile of S1PR modulators. This review focuses on the S1P pathway, the characteristics and S1PR binding profiles of S1PR modulators, the mechanisms of action of S1PR modulators with regard to immune cell trafficking and neuroprotection in MS, together with a summary of the clinical effectiveness of the S1PR modulators that are approved or in late-stage development for patients with MS. Sphingosine 1-phosphate receptor modulator therapy for multiple sclerosis: differential downstream receptor signalling and clinical profile effects (MP4 65540 kb).
Conflict of interest statement
Jerold Chun has received consulting fees or research support from Abbott, AbbVie, Amira, Arena Pharmaceuticals, Biogen Idec, BiolineRX, Blade Therapeutics, Brainstorm Cell Therapeutics, Celgene, GlaxoSmithKline, Inception Sciences, Johnson & Johnson, Merck, Mitsubishi Tanabe, Novartis, Ono Pharmaceuticals, Pfizer, SKAI Ventures and Taisho Pharmaceutical Co. Gavin Giovannoni has received speaker honoraria and consulting fees from AbbVie, Actelion, Almirall, Atara Bio, Bayer Schering Pharma, Biogen Idec, Five Prime, GlaxoSmithKline, GW Pharmaceuticals, Ironwood, Merck & Co., Merck KGaA, Novartis, Pfizer Inc., Protein Discovery Laboratories, Sanofi-Genzyme, Teva Pharmaceutical Industries Ltd, UCB and Vertex Pharmaceuticals. Samuel F. Hunter reports having served as a consultant for AbbVie, Bayer, Genentech/Roche and Sanofi-Genzyme; participation in clinical research trials for Actelion, Adamas, Genentech/Roche, Osmotica and Teva; receipt of research grant support from Sanofi-Genzyme; serving on speakers’ bureaux for Mallinckrodt, Novartis, Sanofi-Genzyme and Teva.
Figures



References
-
- Novartis Pharmaceuticals Corporation. Mayzent®—prescribing information (2019). https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/file.... Accessed 19 Sept 2019.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

