Collective Total Synthesis of Casbane Diterpenes: One Strategy, Multiple Targets
- PMID: 33289954
- PMCID: PMC7986786
- DOI: 10.1002/anie.202015243
Collective Total Synthesis of Casbane Diterpenes: One Strategy, Multiple Targets
Abstract
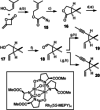
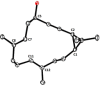
Of the more than 100 casbane diterpenes known to date, only the eponymous parent hydrocarbon casbene itself has ever been targeted by chemical synthesis. Outlined herein is a conceptually new approach that brings not a single but a variety of casbane derivatives into reach, especially the more highly oxygenated and arguably more relevant members of this family. The key design elements are a catalyst-controlled intramolecular cyclopropanation with or without subsequent equilibration, chain extension of the resulting stereoisomeric cyclopropane building blocks by chemoselective hydroboration/cross-coupling, and the efficient closure of the strained macrobicyclic framework by ring-closing alkyne metathesis. A hydroxy-directed catalytic trans-hydrostannation allows for late-stage diversity. These virtues are manifested in the concise total syntheses of depressin, yuexiandajisu A, and ent-pekinenin C. The last compound turned out to be identical to euphorhylonal A, the structure of which had clearly been misassigned.
Keywords: alkyne metathesis; cyclopropanes; hydrostannation; macrocycles; terpenes.
© 2020 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Adolf W., Hecker E., Isr. J. Chem. 1977, 16, 75–83.
-
- Breitmaier E., Terpenes: Flavors, Fragrances, Pharmaca, Pheromones, Wiley-VCH, Weinheim, 2006.
-
- Li Y., Carbone M., Vitale R. M., Amodeo P., Castelluccio F., Sicilia G., Mollo E., Nappo M., Cimino G., Guo Y.-W., Gavagnin M., J. Nat. Prod. 2010, 73, 133–138. - PubMed
-
- Inoue Y., Sakai M., Yao Q., Tanimoto Y., Toshima H., Hasegawa M., Biosci. Biotechnol. Biochem. 2013, 77, 760–765. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

