A Cluster-Randomized Trial of Hydroxychloroquine for Prevention of Covid-19
- PMID: 33289973
- PMCID: PMC7722693
- DOI: 10.1056/NEJMoa2021801
A Cluster-Randomized Trial of Hydroxychloroquine for Prevention of Covid-19
Abstract
Background: Current strategies for preventing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection are limited to nonpharmacologic interventions. Hydroxychloroquine has been proposed as a postexposure therapy to prevent coronavirus disease 2019 (Covid-19), but definitive evidence is lacking.
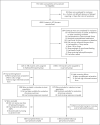
Methods: We conducted an open-label, cluster-randomized trial involving asymptomatic contacts of patients with polymerase-chain-reaction (PCR)-confirmed Covid-19 in Catalonia, Spain. We randomly assigned clusters of contacts to the hydroxychloroquine group (which received the drug at a dose of 800 mg once, followed by 400 mg daily for 6 days) or to the usual-care group (which received no specific therapy). The primary outcome was PCR-confirmed, symptomatic Covid-19 within 14 days. The secondary outcome was SARS-CoV-2 infection, defined by symptoms compatible with Covid-19 or a positive PCR test regardless of symptoms. Adverse events were assessed for up to 28 days.
Results: The analysis included 2314 healthy contacts of 672 index case patients with Covid-19 who were identified between March 17 and April 28, 2020. A total of 1116 contacts were randomly assigned to receive hydroxychloroquine and 1198 to receive usual care. Results were similar in the hydroxychloroquine and usual-care groups with respect to the incidence of PCR-confirmed, symptomatic Covid-19 (5.7% and 6.2%, respectively; risk ratio, 0.86 [95% confidence interval, 0.52 to 1.42]). In addition, hydroxychloroquine was not associated with a lower incidence of SARS-CoV-2 transmission than usual care (18.7% and 17.8%, respectively). The incidence of adverse events was higher in the hydroxychloroquine group than in the usual-care group (56.1% vs. 5.9%), but no treatment-related serious adverse events were reported.
Conclusions: Postexposure therapy with hydroxychloroquine did not prevent SARS-CoV-2 infection or symptomatic Covid-19 in healthy persons exposed to a PCR-positive case patient. (Funded by the crowdfunding campaign YoMeCorono and others; BCN-PEP-CoV2 ClinicalTrials.gov number, NCT04304053.).
Copyright © 2020 Massachusetts Medical Society.
Figures

References
-
- World Health Organization. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). February 2020 (https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mis...).
-
- World Health Organization. Strengthening preparedness for COVID-19 in cities and urban settings. April 2020 (https://www.who.int/publications-detail/strengthening-preparedness-for-c...).
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
