Biomarkers of inflammation and repair in kidney disease progression
- PMID: 33290282
- PMCID: PMC7843225
- DOI: 10.1172/JCI139927
Biomarkers of inflammation and repair in kidney disease progression
Abstract
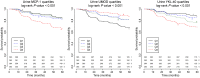
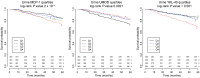
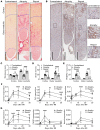
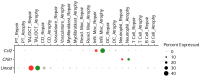
INTRODUCTIONAcute kidney injury and chronic kidney disease (CKD) are common in hospitalized patients. To inform clinical decision making, more accurate information regarding risk of long-term progression to kidney failure is required.METHODSWe enrolled 1538 hospitalized patients in a multicenter, prospective cohort study. Monocyte chemoattractant protein 1 (MCP-1/CCL2), uromodulin (UMOD), and YKL-40 (CHI3L1) were measured in urine samples collected during outpatient follow-up at 3 months. We followed patients for a median of 4.3 years and assessed the relationship between biomarker levels and changes in estimated glomerular filtration rate (eGFR) over time and the development of a composite kidney outcome (CKD incidence, CKD progression, or end-stage renal disease). We paired these clinical studies with investigations in mouse models of renal atrophy and renal repair to further understand the molecular basis of these markers in kidney disease progression.RESULTSHigher MCP-1 and YKL-40 levels were associated with greater eGFR decline and increased incidence of the composite renal outcome, whereas higher UMOD levels were associated with smaller eGFR declines and decreased incidence of the composite kidney outcome. A multimarker score increased prognostic accuracy and reclassification compared with traditional clinical variables alone. The mouse model of renal atrophy showed greater Ccl2 and Chi3l1 mRNA expression in infiltrating macrophages and neutrophils, respectively, and evidence of progressive renal fibrosis compared with the repair model. The repair model showed greater Umod expression in the loop of Henle and correspondingly less fibrosis.CONCLUSIONSBiomarker levels at 3 months after hospitalization identify patients at risk for kidney disease progression.FUNDINGNIH.
Keywords: Chronic kidney disease; Clinical practice; Inflammation; Molecular diagnosis; Nephrology.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL085757/HL/NHLBI NIH HHS/United States
- P30 DK079310/DK/NIDDK NIH HHS/United States
- U01 DK084012/DK/NIDDK NIH HHS/United States
- R01 DK114014/DK/NIDDK NIH HHS/United States
- U01 DK082185/DK/NIDDK NIH HHS/United States
- K01 DK120783/DK/NIDDK NIH HHS/United States
- P30 DK114809/DK/NIDDK NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- K23 DK116967/DK/NIDDK NIH HHS/United States
- U01 DK082183/DK/NIDDK NIH HHS/United States
- R01 DK093771/DK/NIDDK NIH HHS/United States
- R01 DK101507/DK/NIDDK NIH HHS/United States
- R01 DK098233/DK/NIDDK NIH HHS/United States
- R03 DK111881/DK/NIDDK NIH HHS/United States
- U01 DK082192/DK/NIDDK NIH HHS/United States
- UH3 DK114866/DK/NIDDK NIH HHS/United States
- U01 DK082223/DK/NIDDK NIH HHS/United States
- K23 DK100468/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

