Onset hyperalgesia and offset analgesia: Transient increases or decreases of noxious thermal stimulus intensity robustly modulate subsequent perceived pain intensity
- PMID: 33290407
- PMCID: PMC7723268
- DOI: 10.1371/journal.pone.0231124
Onset hyperalgesia and offset analgesia: Transient increases or decreases of noxious thermal stimulus intensity robustly modulate subsequent perceived pain intensity
Abstract
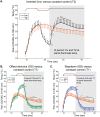
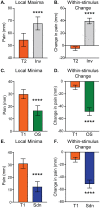
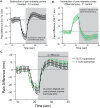
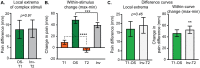
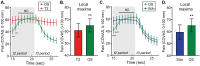
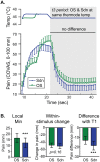
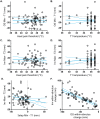
Reported pain intensity depends not only on stimulus intensity but also on previously experienced pain. A painfully hot temperature applied to the skin evokes a lower subjective pain intensity if immediately preceded by a higher temperature, a phenomenon called offset analgesia. Previous work indicated that prior pain experience can also increase subsequent perceived pain intensity. Therefore, we examined whether a given noxious stimulus is experienced as more intense when it is preceded by an increase from a lower temperature. Using healthy volunteer subjects, we observed a disproportionate increase in pain intensity at a given stimulus intensity when this intensity is preceded by a rise from a lower intensity. This disproportionate increase is similar in magnitude to that of offset analgesia. We call this effect onset hyperalgesia. Control stimuli, in which a noxious temperature is held constant, demonstrate that onset hyperalgesia is distinct from receptor or central sensitization. The absolute magnitudes of offset analgesia and onset hyperalgesia correlate with each other but not with the noxious stimulus temperature. Finally, the magnitude of both offset analgesia and onset hyperalgesia depends on preceding temperature changes. Overall, this study demonstrates that the perceptual effect of a noxious thermal stimulus is influenced in a bidirectional manner depending upon both the intensity and direction of change of the immediately preceding thermal stimulus.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Price DD. Psychological and neural mechanisms of pain. New York: Raven Press; 1988. x, 241 p. p.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

