The side effect profile of Clozapine in real world data of three large mental health hospitals
- PMID: 33290433
- PMCID: PMC7723266
- DOI: 10.1371/journal.pone.0243437
The side effect profile of Clozapine in real world data of three large mental health hospitals
Abstract
Objective: Mining the data contained within Electronic Health Records (EHRs) can potentially generate a greater understanding of medication effects in the real world, complementing what we know from Randomised control trials (RCTs). We Propose a text mining approach to detect adverse events and medication episodes from the clinical text to enhance our understanding of adverse effects related to Clozapine, the most effective antipsychotic drug for the management of treatment-resistant schizophrenia, but underutilised due to concerns over its side effects.
Material and methods: We used data from de-identified EHRs of three mental health trusts in the UK (>50 million documents, over 500,000 patients, 2835 of which were prescribed Clozapine). We explored the prevalence of 33 adverse effects by age, gender, ethnicity, smoking status and admission type three months before and after the patients started Clozapine treatment. Where possible, we compared the prevalence of adverse effects with those reported in the Side Effects Resource (SIDER).
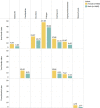
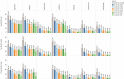
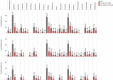
Results: Sedation, fatigue, agitation, dizziness, hypersalivation, weight gain, tachycardia, headache, constipation and confusion were amongst the highest recorded Clozapine adverse effect in the three months following the start of treatment. Higher percentages of all adverse effects were found in the first month of Clozapine therapy. Using a significance level of (p< 0.05) our chi-square tests show a significant association between most of the ADRs and smoking status and hospital admission, and some in gender, ethnicity and age groups in all trusts hospitals. Later we combined the data from the three trusts hospitals to estimate the average effect of ADRs in each monthly interval. In gender and ethnicity, the results show significant association in 7 out of 33 ADRs, smoking status shows significant association in 21 out of 33 ADRs and hospital admission shows the significant association in 30 out of 33 ADRs.
Conclusion: A better understanding of how drugs work in the real world can complement clinical trials.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Effects of six antipsychotic drug treatment regimens on short-term heart rate variability in patients with schizophrenia.Int J Psychiatry Med. 2025 Jul;60(4):363-377. doi: 10.1177/00912174241293650. Epub 2024 Oct 25. Int J Psychiatry Med. 2025. PMID: 39455903
-
Characteristics of adverse reactions among antipsychotic drugs using the Korean Adverse Event Reporting System database from 2010 to 2019.J Psychopharmacol. 2022 Sep;36(9):1041-1050. doi: 10.1177/02698811221104055. Epub 2022 Jun 13. J Psychopharmacol. 2022. PMID: 35695641
-
[Antipsychotics in bipolar disorders].Encephale. 2004 Sep-Oct;30(5):417-24. doi: 10.1016/s0013-7006(04)95456-5. Encephale. 2004. PMID: 15627046 Review. French.
-
Weight gain in children and adolescents during 45 weeks treatment with clozapine, olanzapine and risperidone.J Neural Transm (Vienna). 2008 Nov;115(11):1599-608. doi: 10.1007/s00702-008-0105-9. Epub 2008 Sep 9. J Neural Transm (Vienna). 2008. PMID: 18779922
-
An open, large, 6-month naturalistic study of outcome in schizophrenic outpatients, treated with olanzapine.Hum Psychopharmacol. 2011 Jan;26(1):81-5. doi: 10.1002/hup.1173. Epub 2011 Feb 9. Hum Psychopharmacol. 2011. PMID: 23055416 Review.
Cited by
-
Transient drop in the neutrophil count during COVID-19 regardless of clozapine treatment in patients with mental illness.Rev Psiquiatr Salud Ment (Engl Ed). 2021 Jul 3;15(2):134-7. doi: 10.1016/j.rpsm.2021.06.002. Online ahead of print. Rev Psiquiatr Salud Ment (Engl Ed). 2021. PMID: 34229112 Free PMC article. No abstract available.
-
You lack the season of all natures, sleep.J Clin Sleep Med. 2022 Feb 1;18(2):343-344. doi: 10.5664/jcsm.9826. J Clin Sleep Med. 2022. PMID: 34857085 Free PMC article. No abstract available.
-
Seventy Years of Treating Delusional Disorder with Antipsychotics: A Historical Perspective.Biomedicines. 2022 Dec 18;10(12):3281. doi: 10.3390/biomedicines10123281. Biomedicines. 2022. PMID: 36552037 Free PMC article. Review.
-
Clozapine Efficacy and Adverse Drug Reactions Among a Nationwide Study of 1021 Australians Prescribed Clozapine: The ClozaGene Study.Schizophr Bull. 2025 Mar 14;51(2):458-469. doi: 10.1093/schbul/sbae065. Schizophr Bull. 2025. PMID: 38713070 Free PMC article.
-
Clozapine and Pneumonia: Synthesizing the Link by Reviewing Existing Reports-A Systematic Review and Meta-Analysis.Medicina (Kaunas). 2024 Dec 6;60(12):2016. doi: 10.3390/medicina60122016. Medicina (Kaunas). 2024. PMID: 39768896 Free PMC article.
References
-
- U.S. Food and Drug Administration [Available from: https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Survei....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

