Carbodicarbene Bismaalkene Cations: Unravelling the Complexities of Carbene versus Carbone in Heavy Pnictogen Chemistry
- PMID: 33290596
- PMCID: PMC7986408
- DOI: 10.1002/anie.202014398
Carbodicarbene Bismaalkene Cations: Unravelling the Complexities of Carbene versus Carbone in Heavy Pnictogen Chemistry
Abstract
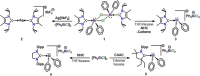
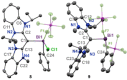
We report a combined experimental and theoretical study on the first examples of carbodicarbene (CDC)-stabilized bismuth complexes, which feature low-coordinate cationic bismuth centers with C=Bi multiple-bond character. Monocations [(CDC)Bi(Ph)Cl][SbF6 ] (8) and [(CDC)BiBr2 (THF)2 ][SbF6 ] (11), dications [(CDC)Bi(Ph)][SbF6 ]2 (9) and [(CDC)BiBr(THF)3 ][NTf2 ]2 (12), and trication [(CDC)2 Bi][NTf2 ]3 (13) have been synthesized via sequential halide abstractions from (CDC)Bi(Ph)Cl2 (7) and (CDC)BiBr3 (10). Notably, the dications and trication exhibit C Bi double dative bonds and thus represent unprecedented bismaalkene cations. The synthesis of these species highlights a unique non-reductive route to C-Bi π-bonding character. The CDC-[Bi] complexes (7-13) were compared with related NHC-[Bi] complexes (1, 3-6) and show substantially different structural properties. Indeed, the CDC ligand has a remarkable influence on the overall stability of the resulting low-coordinate Bi complexes, suggesting that CDC is a superior ligand to NHC in heavy pnictogen chemistry.
Keywords: bismaalkenes; carbenes; carbodicarbenes; cations; low-coordinate compounds.
© 2020 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




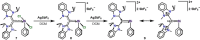



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

