Engineered Sortases in Peptide and Protein Chemistry
- PMID: 33290621
- PMCID: PMC8248031
- DOI: 10.1002/cbic.202000745
Engineered Sortases in Peptide and Protein Chemistry
Abstract
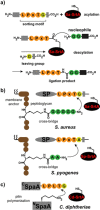
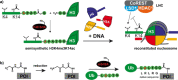
The transpeptidase sortase A of Staphylococcus aureus (Sa-SrtA) is a valuable tool in protein chemistry. The native enzyme anchors surface proteins containing a highly conserved LPxTG sorting motif to a terminal glycine residue of the peptidoglycan layer in Gram-positive bacteria. This reaction is exploited for sortase-mediated ligation (SML), allowing the site-specific linkage of synthetic peptides and recombinant proteins by a native peptide bond. However, the moderate catalytic efficiency and specificity of Sa-SrtA fueled the development of new biocatalysts for SML, including the screening of sortase A variants form microorganisms other than S. aureus and the directed protein evolution of the Sa-SrtA enzyme itself. Novel display platforms and screening formats were developed to isolate sortases with altered properties from mutant libraries. This yielded sortases with strongly enhanced catalytic activity and enzymes recognizing new sorting motifs as substrates. This minireview focuses on recent advances in the field of directed sortase evolution and applications of these tailor-made enzymes in biochemistry.
Keywords: protein bioconjugation; protein engineering; protein semisynthesis; sortases; transpeptidases.
© 2020 The Authors. ChemBioChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

