Hyaluronan synthesis inhibition impairs antigen presentation and delays transplantation rejection
- PMID: 33290836
- PMCID: PMC8147171
- DOI: 10.1016/j.matbio.2020.12.001
Hyaluronan synthesis inhibition impairs antigen presentation and delays transplantation rejection
Abstract
A coat of pericellular hyaluronan surrounds mature dendritic cells (DC) and contributes to cell-cell interactions. We asked whether 4-methylumbelliferone (4MU), an oral inhibitor of HA synthesis, could inhibit antigen presentation. We find that 4MU treatment reduces pericellular hyaluronan, destabilizes interactions between DC and T-cells, and prevents T-cell proliferation in vitro and in vivo. These effects were observed only when 4MU was added prior to initial antigen presentation but not later, consistent with 4MU-mediated inhibition of de novo antigenic responses. Building on these findings, we find that 4MU delays rejection of allogeneic pancreatic islet transplant and allogeneic cardiac transplants in mice and suppresses allogeneic T-cell activation in human mixed lymphocyte reactions. We conclude that 4MU, an approved drug, may have benefit as an adjunctive agent to delay transplantation rejection.
Keywords: 4-methylumbelliferone; Antigen presentation; Dendritic cells; Glycocalyx; Hyaluronan; Transplantation.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors have no competing financial interests. N.N., P.L.M., and P.L.B. are listed as inventors of the patent (PCT/US2019/019310) filed by the Board of Trustees of the Leland Stanford Junior University.
Figures
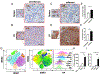


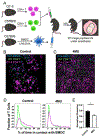

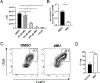
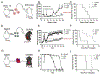
References
-
- Ito S., Structure and function of the glycocalyx., Fed. Proc 28 (n.d.) 12–25. http://www.ncbi.nlm.nih.gov/pubmed/4884661. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

