Bioactive sphingolipids: Advancements and contributions from the laboratory of Dr. Lina M. Obeid
- PMID: 33290840
- PMCID: PMC8244749
- DOI: 10.1016/j.cellsig.2020.109875
Bioactive sphingolipids: Advancements and contributions from the laboratory of Dr. Lina M. Obeid
Abstract
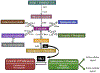
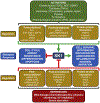
Sphingolipids and their synthetic enzymes have emerged as critical mediators in numerous diseases including inflammation, aging, and cancer. One enzyme in particular, sphingosine kinase (SK) and its product sphingosine-1-phosphate (S1P), has been extensively implicated in these processes. SK catalyzes the phosphorylation of sphingosine to S1P and exists as two isoforms, SK1 and SK2. In this review, we will discuss the contributions from the laboratory of Dr. Lina M. Obeid that have defined the roles for several bioactive sphingolipids in signaling and disease with an emphasis on her work defining SK1 in cellular fates and pathobiologies including proliferation, senescence, apoptosis, and inflammation.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Hannun YA, Obeid LM, Principles of bioactive lipid signalling: lessons from sphingolipids, Nat. Rev. Mol. Cell Biol 9 (2008) 139–150. - PubMed
-
- Zitomer NC, Mitchell T, Voss KA, Bondy GS, Pruett ST, Gamier-Amblard EC, Liebeskind LS, Park H, Wang E, Sullards MC, Merrill AH Jr., Riley RT, Ceramide synthase inhibition by fumonisin B1 causes accumulation of 1-deoxysphinganine: a novel category of bioactive 1-deoxysphingoid bases and 1-deoxydihydroceramides biosynthesized by mammalian cell lines and animals, J. Biol. Chem 284 (2009) 4786–4795. - PMC - PubMed
-
- Anelli V, Gault CR, Cheng AB, Obeid LM, Sphingosine Kinase 1 Is Upregulated during Hypoxia in U87MG glioma cells: role of Hypoxia-inducible factors 1 and 2, J. Biol. Chem 283 (2008) 3365–3375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

