Fatty acid binding protein-4 promotes alcohol-dependent hepatosteatosis and hepatocellular carcinoma progression
- PMID: 33290990
- PMCID: PMC7719965
- DOI: 10.1016/j.tranon.2020.100975
Fatty acid binding protein-4 promotes alcohol-dependent hepatosteatosis and hepatocellular carcinoma progression
Abstract
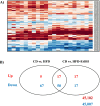
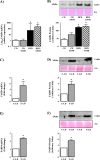
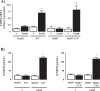
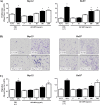
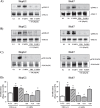
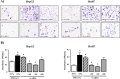
Fatty liver disease (hepatosteatosis) is a common early pathology in alcohol-dependent and obese patients. Fatty acid binding protein-4 (FABP4) is normally expressed in adipocytes and macrophages and functions as a regulator of intracellular lipid movement/storage. This study sought to investigate hepatic FABP4 expression and function in alcoholic liver disease (ALD) and hepatocellular carcinoma (HCC). Using chronic ethanol fed mouse models and patient samples FABP4 expression was analyzed. Human HCC cells, and HCC cells transfected to express CYP2E1, were exposed to ethanol and analyzed for FABP4 expression, or exposed to rhFABP4 (in the absence/presence of ERK, p38-MAPK or JNK1/2 inhibitors) and cell proliferation and migration measured. Hepatosteatotic-ALD mouse models exhibited increased hepatic FABP4 mRNA and protein levels, with FABP4 expression confirmed in hepatocytes. In HCC cells, CYP2E1-dependent ethanol metabolism induced FABP4 expression in vitro and exogenous rhFABP4 stimulated proliferation and migration, effects abrogated by ERK and JNK1/2 inhibition. Increased FABP4 was also detected in ALD/ALD-HCC patients, but not patients with viral hepatitis/HCC. Collectively these data demonstrate ethanol metabolism induces hepatic FABP4 expression and FABP4 promotes hepatoma cell proliferation/migration. These data suggest liver-derived FABP4 may be an important paracrine-endocrine factor during hepatic foci expansion and/or hepatoma progression in the underlying setting of ALD.
Keywords: Alcohol; Fatty acid binding protein 4; Hepatocellular carcinoma; Hepatosteatosis; Liver.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest None of the authors have any conflicts of interest to declare.
Figures

References
-
- McKillop I.H., Girardi C.A., Thompson K.J. Role of fatty acid binding proteins (FABPs) in cancer development and progression. Cell Signal. 2019;62 - PubMed
-
- Sagnelli E., Macera M., Russo A., Coppola N., Sagnelli C. Epidemiological and etiological variations in hepatocellular carcinoma. Infection. 2020;48:7–17. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

