Intravitreal Injection of Liposomes Loaded with a Histone Deacetylase Inhibitor Promotes Retinal Ganglion Cell Survival in a Mouse Model of Optic Nerve Crush
- PMID: 33291226
- PMCID: PMC7730870
- DOI: 10.3390/ijms21239297
Intravitreal Injection of Liposomes Loaded with a Histone Deacetylase Inhibitor Promotes Retinal Ganglion Cell Survival in a Mouse Model of Optic Nerve Crush
Abstract
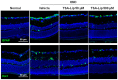
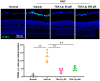
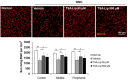
Various neuroprotective agents have been studied for the treatment of retinal ganglion cell (RGC) diseases, but issues concerning the side effects of systemically administered drugs and the short retention time of intravitreally injected drugs limit their clinical applications. The current study aimed to evaluate the neuroprotective effects of intravitreally injected trichostatin A (TSA)-loaded liposomes in a mouse model of optic nerve crush (ONC) and determine whether TSA-loaded liposomes have therapeutic potential in RGC diseases. The histone deacetylase inhibitor, TSA, was incorporated into polyethylene glycolylated liposomes. C57BL/6J mice were treated with an intravitreal injection of TSA-loaded liposomes and liposomes loaded with a lipophilic fluorescent dye for tracking, immediately after ONC injury. The expression of macroglial and microglial cell markers (glial fibrillary acidic protein and ionized calcium binding adaptor molecule-1), RGC survival, and apoptosis were assessed. We found that the liposomes reached the inner retina. Their fluorescence was detected for up to 10 days after the intravitreal injection, with peak intensity at 3 days postinjection. Intravitreally administered TSA-loaded liposomes significantly decreased reactive gliosis and RGC apoptosis and increased RGC survival in a mouse model of ONC. Our results suggest that TSA-loaded liposomes may help in the treatment of various RGC diseases.
Keywords: histone deacetylase inhibitor; intravitreal injection; liposome; optic nerve crush; retinal ganglion cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Targeting HDAC3 Activity with RGFP966 Protects Against Retinal Ganglion Cell Nuclear Atrophy and Apoptosis After Optic Nerve Injury.J Ocul Pharmacol Ther. 2018 Apr;34(3):260-273. doi: 10.1089/jop.2017.0059. Epub 2017 Dec 6. J Ocul Pharmacol Ther. 2018. PMID: 29211617 Free PMC article.
-
Effect of intravitreal injection of bevacizumab on optic nerve head leakage and retinal ganglion cell survival in a mouse model of optic nerve crush.Invest Ophthalmol Vis Sci. 2013 Dec 17;54(13):8160-71. doi: 10.1167/iovs.13-12771. Invest Ophthalmol Vis Sci. 2013. PMID: 24168994
-
Neuroprotective Effects of Human Serum Albumin Nanoparticles Loaded With Brimonidine on Retinal Ganglion Cells in Optic Nerve Crush Model.Invest Ophthalmol Vis Sci. 2015 Aug;56(9):5641-9. doi: 10.1167/iovs.15-16538. Invest Ophthalmol Vis Sci. 2015. PMID: 26313300
-
Ocular Effects of Sildenafil in Naïve Mice and a Mouse Model of Optic Nerve Crush.Invest Ophthalmol Vis Sci. 2019 May 1;60(6):1987-1995. doi: 10.1167/iovs.18-26333. Invest Ophthalmol Vis Sci. 2019. PMID: 31063183
-
High-Mobility Group Box 1 Inhibitor BoxA Alleviates Neuroinflammation-Induced Retinal Ganglion Cell Damage in Traumatic Optic Neuropathy.Int J Mol Sci. 2022 Jun 16;23(12):6715. doi: 10.3390/ijms23126715. Int J Mol Sci. 2022. PMID: 35743157 Free PMC article.
Cited by
-
Use of gene therapy for optic nerve protection: Current concepts.Front Neurosci. 2023 Apr 6;17:1158030. doi: 10.3389/fnins.2023.1158030. eCollection 2023. Front Neurosci. 2023. PMID: 37090805 Free PMC article. Review.
-
Nanocarriers for the Delivery of Neuroprotective Agents in the Treatment of Ocular Neurodegenerative Diseases.Pharmaceutics. 2023 Mar 3;15(3):837. doi: 10.3390/pharmaceutics15030837. Pharmaceutics. 2023. PMID: 36986699 Free PMC article. Review.
-
Looking to the Future of Viral Vectors in Ocular Gene Therapy: Clinical Review.Biomedicines. 2025 Feb 5;13(2):365. doi: 10.3390/biomedicines13020365. Biomedicines. 2025. PMID: 40002778 Free PMC article. Review.
-
A New Era in Ocular Therapeutics: Advanced Drug Delivery Systems for Uveitis and Neuro-Ophthalmologic Conditions.Pharmaceutics. 2023 Jul 14;15(7):1952. doi: 10.3390/pharmaceutics15071952. Pharmaceutics. 2023. PMID: 37514137 Free PMC article. Review.
-
Overcoming Treatment Challenges in Posterior Segment Diseases with Biodegradable Nano-Based Drug Delivery Systems.Pharmaceutics. 2023 Mar 29;15(4):1094. doi: 10.3390/pharmaceutics15041094. Pharmaceutics. 2023. PMID: 37111579 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

