Impact of Adverse Drug Reactions in Patients with End Stage Renal Disease in Greece
- PMID: 33291233
- PMCID: PMC7730015
- DOI: 10.3390/ijerph17239101
Impact of Adverse Drug Reactions in Patients with End Stage Renal Disease in Greece
Abstract
Background: Patients with end-stage renal disease (ESRD) require specialized therapeutic interventions. The decreased renal function that modulates the physiology and presence of comorbidities is often associated with variations in the pharmacological response, thus increasing the risk of adverse drug events or reactions (ADE/ADRs) from co-administered drugs.
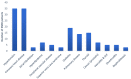
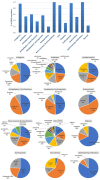
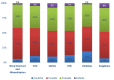
Methods: A cross-sectional study to record comorbidities, drug-drug interactions (DDIs), ADE/ADRs in patients with chronic kidney disease of stage five in Greece. The study enrolled 60 patients of mean age 64.8 ± 12.9 years, undergoing hemodialysis three times a week. Demographic and social factors, comorbidities, laboratory test data, medication regimens, DDIs and the reporting of ADE/ADRs were analyzed.
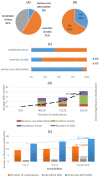
Results: Cardiovascular diseases and diabetes were the main comorbidities. In total, 50 different DDIs of various clinical significance were identified. CNS, GI-track, and musculoskeletal-system-related ADE/ADRs were most often reported by patients. ADE/ADRs as clinical outcome from DDIs were associated in 64% of the total identified DDIs. There was a positive trend between number of medications, ADE/ADRs report and DDIs.
Conclusions: The impact of ADE/ADRs in ESRD patients should be always considered. Guidelines as well as continuous training in the context of evidence-based clinical practice by healthcare personnel on therapy administration and prevention of adverse events are important.
Keywords: adverse drug reactions; chronic disease; chronic kidney disease; drug–drug interactions; end-stage renal disease; quality of life.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Bikbov B., Purcell C.A., Levey A.S., Smith M., Abdoli A., Abebe M., Adebayo O.M., Afarideh M., Agarwal S.K., Agudelo-Botero M., et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. - DOI - PMC - PubMed
-
- Seckinger J., Dschietzig W., Leimenstoll G., Rob P.M., Kuhlmann M.K., Pommer W., Fraass U., Ritz E., Schwenger V. Morbidity, mortality and quality of life in the ageing haemodialysis population: Results from the ELDERLY study. Clin. Kidney J. 2016;9:839–848. doi: 10.1093/ckj/sfw087. - DOI - PMC - PubMed
-
- Sombolos K., Tsakiris D., Boletis J., Vlahakos D., Siamopoulos K.C., Vargemezis V., Nikolaidis P., Iatrou C., Dafnis E., Xynos K., et al. Multicenter epidemiological study to assess the population of CKD patients in Greece: Results from the PRESTAR study. PLoS ONE. 2014;9:e112767. doi: 10.1371/journal.pone.0112767. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

