The Roles of Sodium-Independent Inorganic Phosphate Transporters in Inorganic Phosphate Homeostasis and in Cancer and Other Diseases
- PMID: 33291240
- PMCID: PMC7729900
- DOI: 10.3390/ijms21239298
The Roles of Sodium-Independent Inorganic Phosphate Transporters in Inorganic Phosphate Homeostasis and in Cancer and Other Diseases
Abstract
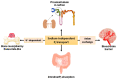
Inorganic phosphate (Pi) is an essential nutrient for the maintenance of cells. In healthy mammals, extracellular Pi is maintained within a narrow concentration range of 0.70 to 1.55 mM. Mammalian cells depend on Na+/Pi cotransporters for Pi absorption, which have been well studied. However, a new type of sodium-independent Pi transporter has been identified. This transporter assists in the absorption of Pi by intestinal cells and renal proximal tubule cells and in the reabsorption of Pi by osteoclasts and capillaries of the blood-brain barrier (BBB). Hyperphosphatemia is a risk factor for mineral deposition, the development of diseases such as osteoarthritis, and vascular calcifications (VCs). Na+-independent Pi transporters have been identified and biochemically characterized in vascular smooth muscle cells (VSMCs), chondrocytes, and matrix vesicles, and their involvement in mineral deposition in the extracellular microenvironment has been suggested. According to the growth rate hypothesis, cancer cells require more phosphate than healthy cells due to their rapid growth rates. Recently, it was demonstrated that breast cancer cells (MDA-MB-231) respond to high Pi concentration (2 mM) by decreasing Na+-dependent Pi transport activity concomitant with an increase in Na+-independent (H+-dependent) Pi transport. This Pi H+-dependent transport has a fundamental role in the proliferation and migratory capacity of MDA-MB-231 cells. The purpose of this review is to discuss experimental findings regarding Na+-independent inorganic phosphate transporters and summarize their roles in Pi homeostasis, cancers and other diseases, such as osteoarthritis, and in processes such as VC.
Keywords: cancer; inorganic phosphate; inorganic phosphate homeostasis; sodium-independent Pi transport.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

