T Cell Immunity and the Quest for Protective Vaccines against Staphylococcus aureus Infection
- PMID: 33291260
- PMCID: PMC7762175
- DOI: 10.3390/microorganisms8121936
T Cell Immunity and the Quest for Protective Vaccines against Staphylococcus aureus Infection
Abstract
Staphylococcus aureus is a wide-spread human pathogen, and one of the top causative agents of nosocomial infections. The prevalence of antibiotic-resistant S. aureus strains, which are associated with higher mortality and morbidity rates than antibiotic-susceptible strains, is increasing around the world. Vaccination would be an effective preventive measure against S. aureus infection, but to date, every vaccine developed has failed in clinical trials, despite inducing robust antibody responses. These results suggest that induction of humoral immunity does not suffice to confer protection against the infection. Evidence from studies in murine models and in patients with immune defects support a role of T cell-mediated immunity in protective responses against S. aureus. Here, we review the current understanding of the mechanisms underlying adaptive immunity to S. aureus infections and discuss these findings in light of the recent S. aureus vaccine trial failures. We make the case for the need to develop anti-S. aureus vaccines that can specifically elicit robust and durable protective memory T cell subsets.
Keywords: Staphylococcus aureus; T cell-mediated immunity; antibodies; tissue-resident memory T cells; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
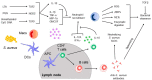
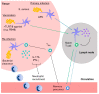
References
-
- Anderson D.J., Sexton D.J., Kanafani Z.A., Auten G., Kaye K.S. Severe surgical site infection in community hospitals: Epidemiology, key procedures, and the changing prevalence of methicillin-resistant Staphylococcus aureus. Infect. Control. Hosp. Epidemiol. 2007;28:1047–1053. doi: 10.1086/520731. - DOI - PubMed
-
- Berríos-Torres S.I., Umscheid C.A., Bratzler D.W., Leas B., Stone E.C., Kelz R.R., Reinke C.E., Morgan S., Solomkin J.S., Mazuki J.E., et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection. JAMA Surg. 2017;152:784–791. doi: 10.1001/jamasurg.2017.0904. - DOI - PubMed
-
- Lee B.Y., Singh A., David M.Z., Bartsch S.M., Slayton R.B., Huang S.S., Zimmer S.M., Potter M.A., Macal C.M., Lauderdale D.S., et al. The economic burden of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) Clin. Microbiol. Infect. 2013;19:528–536. doi: 10.1111/j.1469-0691.2012.03914.x. - DOI - PMC - PubMed
-
- Acton D.S., Plat-Sinnige M.J., van Wamel W., de Groot N., van Belkum A. Intestinal carriage of Staphylococcus aureus: How does its frequency compare with that of nasal carriage and what is its clinical impact? Eur. J. Clin. Microbiol. Infect. Dis. 2009;28:115–127. doi: 10.1007/s10096-008-0602-7. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

