Development and Validation of High-Throughput Bioanalytical Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) Method for the Quantification of Newly Synthesized Antitumor Carbonic Anhydrase Inhibitors in Human Plasma
- PMID: 33291270
- PMCID: PMC7730089
- DOI: 10.3390/molecules25235753
Development and Validation of High-Throughput Bioanalytical Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) Method for the Quantification of Newly Synthesized Antitumor Carbonic Anhydrase Inhibitors in Human Plasma
Abstract
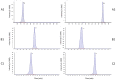
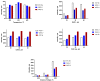
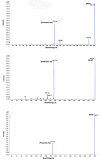
In the present study, a sensitive and fully validated bioanalytical high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) method has been developed for the quantitative determination of three newly synthesized carbonic anhydrases inhibitors (CAIs) with potential antitumor activity in human plasma. The analytes and the internal standard (IS) were extracted using 1.5 mL acetonitrile from only 450 µL aliquots of human plasma to achieve the desired protein precipitation. Chromatographic separations were achieved on Phenomenex Kinetex® C18 column (100 × 4.6 mm, 2.6 µm) using a binary gradient elution mode with a run time of less than 6 min. The mobile phase consisted of solvent (A): 0.1% formic acid in 50% methanol and solvent B: 0.1% formic acid in acetonitrile (30:70, v/v), pumped at a flow rate of 0.8 mL/min. Detection was employed using triple quadrupole tandem mass spectrometer (API 3500) equipped with an electrospray ionization (ESI) source in the positive ion mode. Multiple reaction monitoring (MRM) mode was selected for quantitation through monitoring the precursor-to-parent ion transition at m/z 291.9 → 173.0, m/z 396.9 → 225.1, m/z 388.9 → 217.0, and m/z 146.9 → 91.0 for AW-9a, WES-1, WES-2, and Coumarin (IS), respectively. Linearity was computed using the weighted least-squares linear regression method (1/x2) over a concentration range of 1-1000, 2.5-800, and 5-500 ng/mL for AW-9a, WES-1, and WES-2; respectively. The bioanalytical LC-MS/MS method was fully validated as per U.S. Food and Drug Administration (FDA) guidelines with all respect to linearity, accuracy, precision, carry-over, selectivity, dilution integrity, and stability. The proposed LC-MS/MS method was applied successfully for the determination of all investigated drugs in spiked human plasma with no significant matrix effect, which is a crucial cornerstone in further therapeutic drug monitoring of newly developed therapeutic agents.
Keywords: LC-MS/MS; antitumor; bioanalytical validation; carbonic anhydrase inhibitors; human plasma; tandem mass.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Hilvo M., Baranauskiene L., Salzano A.M., Scaloni A., Matulis D., Innocenti A., Scozzafava A., Monti S.M., Di Fiore A., De Simone G., et al. Biochemical Characterization of CA IX, One of the Most Active Carbonic Anhydrase Isozymes. J. Biol. Chem. 2008;283:27799–27809. doi: 10.1074/jbc.M800938200. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

