Dihydrotanshinone, a Natural Diterpenoid, Preserves Blood-Retinal Barrier Integrity via P2X7 Receptor
- PMID: 33291318
- PMCID: PMC7730037
- DOI: 10.3390/ijms21239305
Dihydrotanshinone, a Natural Diterpenoid, Preserves Blood-Retinal Barrier Integrity via P2X7 Receptor
Abstract
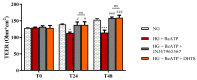
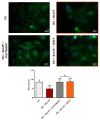
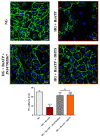
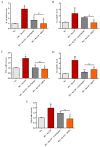
Activation of P2X7 signaling, due to high glucose levels, leads to blood retinal barrier (BRB) breakdown, which is a hallmark of diabetic retinopathy (DR). Furthermore, several studies report that high glucose (HG) conditions and the related activation of the P2X7 receptor (P2X7R) lead to the over-expression of pro-inflammatory markers. In order to identify novel P2X7R antagonists, we carried out virtual screening on a focused compound dataset, including indole derivatives and natural compounds such as caffeic acid phenethyl ester derivatives, flavonoids, and diterpenoids. Molecular Mechanics/Generalized Born Surface Area (MM/GBSA) rescoring and structural fingerprint clustering of docking poses from virtual screening highlighted that the diterpenoid dihydrotanshinone (DHTS) clustered with the well-known P2X7R antagonist JNJ47965567. A human-based in vitro BRB model made of retinal pericytes, astrocytes, and endothelial cells was used to assess the potential protective effect of DHTS against HG and 2'(3')-O-(4-Benzoylbenzoyl)adenosine-5'-triphosphate (BzATP), a P2X7R agonist, insult. We found that HG/BzATP exposure generated BRB breakdown by enhancing barrier permeability (trans-endothelial electrical resistance (TEER)) and reducing the levels of ZO-1 and VE-cadherin junction proteins as well as of the Cx-43 mRNA expression levels. Furthermore, HG levels and P2X7R agonist treatment led to increased expression of pro-inflammatory mediators (TLR-4, IL-1β, IL-6, TNF-α, and IL-8) and other molecular markers (P2X7R, VEGF-A, and ICAM-1), along with enhanced production of reactive oxygen species. Treatment with DHTS preserved the BRB integrity from HG/BzATP damage. The protective effects of DHTS were also compared to the validated P2X7R antagonist, JNJ47965567. In conclusion, we provided new findings pointing out the therapeutic potential of DHTS, which is an inhibitor of P2X7R, in terms of preventing and/or counteracting the BRB dysfunctions elicited by HG conditions.
Keywords: blood-retinal barrier; diabetic retinopathy; endothelial cells; inflammation; oxidative stress; purinergic P2X7 receptor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

