Correlation between Microstructure and Chemical Composition of Zinc Oxide Gas Sensor Layers and Their Gas-Sensitive Properties in Chlorine Atmosphere
- PMID: 33291379
- PMCID: PMC7730158
- DOI: 10.3390/s20236951
Correlation between Microstructure and Chemical Composition of Zinc Oxide Gas Sensor Layers and Their Gas-Sensitive Properties in Chlorine Atmosphere
Abstract
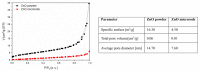
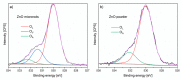
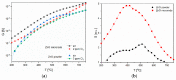
In this article, we present results concerning the impact of structural and chemical properties of zinc oxide in various morphological forms and its gas-sensitive properties, tested in an atmosphere containing a very aggressive gas such as chlorine. The aim of this research was to understand the mechanism of chlorine detection using a resistive gas sensor with an active layer made of zinc oxide with a different structure and morphology. Two types of ZnO sensor layers obtained by two different technological methods were used in sensor construction. Their morphology, crystal structure, specific surface area, porosity, surface chemistry and structural defects were characterized, and then compared with gas-sensitive properties in a chlorine-containing atmosphere. To achieve this goal, scanning electron microscopy (SEM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS) and photoluminescence spectroscopy (PL) methods were used. The sensing properties of obtained active layers were tested by the temperature stimulated conductance method (TSC). We have noticed that their response in a chlorine atmosphere is not determined by the size of the specific surface or porosity. The obtained results showed that the structural defects of ZnO crystals play the most important role in chlorine detection. We demonstrated that Cl2 adsorption is a concurrent process to oxygen adsorption. Both of them occur on the same active species (oxygen vacancies). Their concentration is higher on the side planes of the zinc oxide crystal than the others. Additionally, ZnO sublimation process plays an important role in the chlorine detection mechanism.
Keywords: chemistry; chlorine sensitivity; microstructure; resistive gas sensors; zinc oxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
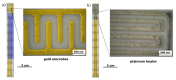







References
-
- Williams D.E. Semiconducting oxides as gas-sensitive resistors. Sens. Actuators B Chem. 1999;57:1–16. doi: 10.1016/S0925-4005(99)00133-1. - DOI
-
- Urasinska-Wojcik B., Vincent T.A., Chowdhury M.F., Gardner J.W. Ultrasensitive WO3 gas sensors for NO2 detection in air and low oxygen environment. Sens. Actuators B Chem. 2017;239:1051–1059. doi: 10.1016/j.snb.2016.08.080. - DOI
-
- Wei H.L., Kumar P., Yao D.J. Printed Resistive Sensor Array Combined with a Flexible Substrate for Ethanol and Methane Detection. ECS J. Solid State Sci. Technol. 2020:115008. doi: 10.1149/2162-8777/ab9fe6. - DOI
-
- Xia Y., Wang J., Xu L., Li X., Huang S. A room-temperature methane sensor based on Pd-decorated ZnO/rGO hybrids enhanced by visible light photocatalysis. Sens. Actuators B Chem. 2020;304:127334. doi: 10.1016/j.snb.2019.127334. - DOI
-
- Latyshev V.M., Berestok T.O., Opanasyuk A.S., Kornyushchenko A.S., Perekrestov V.I. Nanostructured ZnO films for potential use in LPG gas sensors. Solid State Sci. 2017;67:109–113. doi: 10.1016/j.solidstatesciences.2017.02.010. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

