Guanosine-Mediated Anxiolytic-Like Effect: Interplay with Adenosine A1 and A2A Receptors
- PMID: 33291390
- PMCID: PMC7729560
- DOI: 10.3390/ijms21239281
Guanosine-Mediated Anxiolytic-Like Effect: Interplay with Adenosine A1 and A2A Receptors
Abstract
Acute or chronic administration of guanosine (GUO) induces anxiolytic-like effects, for which the adenosine (ADO) system involvement has been postulated yet without a direct experimental evidence. Thus, we aimed to investigate whether adenosine receptors (ARs) are involved in the GUO-mediated anxiolytic-like effect, evaluated by three anxiety-related paradigms in rats. First, we confirmed that acute treatment with GUO exerts an anxiolytic-like effect. Subsequently, we investigated the effects of pretreatment with ADO or A1R (CPA, CCPA) or A2AR (CGS21680) agonists 10 min prior to GUO on a GUO-induced anxiolytic-like effect. All the combined treatments blocked the GUO anxiolytic-like effect, whereas when administered alone, each compound was ineffective as compared to the control group. Interestingly, the pretreatment with nonselective antagonist caffeine or selective A1R (DPCPX) or A2AR (ZM241385) antagonists did not modify the GUO-induced anxiolytic-like effect. Finally, binding assay performed in hippocampal membranes showed that [3H]GUO binding became saturable at 100-300 nM, suggesting the existence of a putative GUO binding site. In competition experiments, ADO showed a potency order similar to GUO in displacing [3H]GUO binding, whereas AR selective agonists, CPA and CGS21680, partially displaced [3H]GUO binding, but the sum of the two effects was able to displace [3H]GUO binding to the same extent of ADO alone. Overall, our results strengthen previous data supporting GUO-mediated anxiolytic-like effects, add new evidence that these effects are blocked by A1R and A2AR agonists and pave, although they do not elucidate the mechanism of GUO and ADO receptor interaction, for a better characterization of GUO binding sites in ARs.
Keywords: A1R; A2AR; adenosine; behavior; caffeine; guanosine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



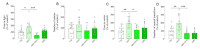

References
-
- Di Liberto V., Mudò G., Garozzo R., Frinchi M., Fernandez-Dueñas V., Di Iorio P., Ciccarelli R., Caciagli F., Condorelli D.F., Ciruela F., et al. The Guanine-Based Purinergic System: The Tale of an Orphan Neuromodulation. Front. Pharmacol. 2016;7:158. doi: 10.3389/fphar.2016.00158. - DOI - PMC - PubMed

