Propagation and Maintenance of Cancer Stem Cells: A Major Influence of the Long Non-Coding RNA H19
- PMID: 33291403
- PMCID: PMC7762009
- DOI: 10.3390/cells9122613
Propagation and Maintenance of Cancer Stem Cells: A Major Influence of the Long Non-Coding RNA H19
Abstract
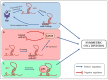
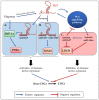
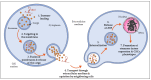
Cancer stem cells (CSCs) represent a rare population of tumor cells that exhibit stem cell properties with the abilities of self-renewal and differentiation. These cells are now widely accepted to be responsible for tumor initiation, development, resistance to conventional therapies, and recurrence. Thus, a better understanding of the molecular mechanisms involved in the control of CSCs is essential to improve patient management in terms of diagnostics and therapies. CSCs are regulated by signals of the tumor microenvironment as well as intrinsic genetic and epigenetic modulators. H19, the first identified lncRNA is involved in the development and progression of many different cancer types. Recently, H19 has been demonstrated to be implicated in the regulation of CSCs in different types of cancers. The aim of this review is to provide an overview of the role and mechanisms of action of H19 in the regulation of CSCs. We summarize how H19 may regulate CSC division and cancer cell reprogramming, thus affecting metastasis and drug resistance. We also discuss the potential clinical implications of H19.
Keywords: H19; cancer stem cells; exosomes; lncRNA; non-coding RNA; reprogramming factors; tumorigenicity.
Conflict of interest statement
The authors declare no conflict of interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

