Impact of Total Neoadjuvant Therapy vs. Standard Chemoradiotherapy in Locally Advanced Rectal Cancer: A Systematic Review and Meta-Analysis of Randomized Trials
- PMID: 33291454
- PMCID: PMC7762140
- DOI: 10.3390/cancers12123655
Impact of Total Neoadjuvant Therapy vs. Standard Chemoradiotherapy in Locally Advanced Rectal Cancer: A Systematic Review and Meta-Analysis of Randomized Trials
Abstract
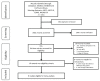
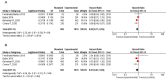
Multimodality treatment is a standard of care for LARC, but the optimal sequencing of the treatment modalities remains unclear. Several randomized clinical trials (RCTs) compared total neoadjuvant treatment (TNT) vs. standard neoadjuvant chemoradiotherapy (CRT) with inconsistent results. A systematic review and meta-analysis was performed to evaluate the efficacy of TNT in terms of complete pathological response (pCR) rate, disease-free and overall survival vs. standard CRT in LARC. A systematic search was performed through MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials and meeting abstracts up to May 2020. RCTs comparing CRT vs. TNT followed by surgery in LARC were eligible for the study. Study selection and data extraction were done following PRISMA guidelines by two independent reviewers. The Mantel-Haenzel method was used to obtain a fixed-effects model of pooled odds or hazard ratios for the main outcomes. Eight RCTs, including 2301 patients, met the eligibility criteria. TNT significantly improved pCR rate (OR = 1.99, 95% confidence interval (CI) 1.59-2.49; p < 0.001), 3-year disease-free-survival (DFS) (HR = 0.82, 95%CI 0.71-0.95; p = 0.01) and 3-year overall survival (OS) (hazard ratio (HR) = 0.81, p = 0.04). Grade 3-4 adverse events were not significantly different in both strategies (OR = 1.58; p = 0.14). An improved pCR rate was documented regardless of the type of radiotherapy administered (long vs. short fractionation schedules). No significant heterogeneity was found. The results of this meta-analysis show that TNT improves pCR and survival rates vs. standard preoperative CRT in patients with LARC. TNT may become a new standard of care in LARC, although longer follow-up is needed to properly assess its long-term impact on survival.
Keywords: chemotherapy; meta-analysis; neoadjuvant therapy; rectal cancer; treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Total Neoadjuvant Therapy (TNT) versus Standard Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer: A Systematic Review and Meta-Analysis.Oncologist. 2021 Sep;26(9):e1555-e1566. doi: 10.1002/onco.13824. Epub 2021 Jun 7. Oncologist. 2021. PMID: 33987952 Free PMC article.
-
Total Neoadjuvant Therapy vs Standard Therapy in Locally Advanced Rectal Cancer: A Systematic Review and Meta-analysis.JAMA Netw Open. 2020 Dec 1;3(12):e2030097. doi: 10.1001/jamanetworkopen.2020.30097. JAMA Netw Open. 2020. PMID: 33326026 Free PMC article.
-
Total neoadjuvant therapy or standard chemoradiotherapy for locally advanced rectal cancer: A systematic review and meta-analysis.Front Surg. 2022 Aug 26;9:911538. doi: 10.3389/fsurg.2022.911538. eCollection 2022. Front Surg. 2022. PMID: 36090336 Free PMC article. Review.
-
Efficacy and safety of total neoadjuvant therapy in locally advanced rectal cancer: a meta-analysis.Int J Colorectal Dis. 2023 Apr 1;38(1):89. doi: 10.1007/s00384-023-04376-y. Int J Colorectal Dis. 2023. PMID: 37004572 Review.
-
Is Total Neoadjuvant Treatment Beneficial for Locally Advanced Rectal Cancer? A Meta-Analysis of Randomized Controlled Trials.Oncology. 2024;102(5):399-413. doi: 10.1159/000534815. Epub 2023 Nov 3. Oncology. 2024. PMID: 37926087
Cited by
-
Neoadjuvant short-course radiotherapy or chemoradiation plus consolidative chemotherapy followed by radical operation for locally advanced rectal cancer.Front Oncol. 2024 Jan 23;13:1284569. doi: 10.3389/fonc.2023.1284569. eCollection 2023. Front Oncol. 2024. PMID: 38322287 Free PMC article.
-
Debating Pros and Cons of Total Neoadjuvant Therapy in Rectal Cancer.Cancers (Basel). 2021 Dec 18;13(24):6361. doi: 10.3390/cancers13246361. Cancers (Basel). 2021. PMID: 34944980 Free PMC article.
-
Survival landscape of different tumor regression grades and pathologic complete response in rectal cancer after neoadjuvant therapy based on reconstructed individual patient data.BMC Cancer. 2021 Nov 13;21(1):1214. doi: 10.1186/s12885-021-08922-1. BMC Cancer. 2021. PMID: 34773999 Free PMC article.
-
Local excision vs. proctectomy in patients with ypT0-1 rectal cancer following neoadjuvant therapy: a propensity score matched analysis of the National Cancer Database.Tech Coloproctol. 2024 Sep 21;28(1):128. doi: 10.1007/s10151-024-02994-4. Tech Coloproctol. 2024. PMID: 39305380 Free PMC article.
-
SMAD3 Host and Tumor Profiling to Identify Locally Advanced Rectal Cancer Patients at High Risk of Poor Response to Neoadjuvant Chemoradiotherapy.Front Pharmacol. 2021 Dec 24;12:778781. doi: 10.3389/fphar.2021.778781. eCollection 2021. Front Pharmacol. 2021. PMID: 35002714 Free PMC article.
References
-
- Peeters K.C.M.J., Marijnen C.A.M., Nagtegaal I.D., Kranenbarg E.K., Putter H., Wiggers T., Rutten H., Pahlman L., Glimelius B., Leer J.W., et al. The TME trial after a median follow-up of 6 years: Increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann. Surg. 2007;246:693–701. doi: 10.1097/01.sla.0000257358.56863.ce. - DOI - PubMed
-
- Sebag-Montefiore D., Stephens R.J., Steele R., Monson J., Grieve R., Khanna S., Quirke P., Couture J., De Metz C., Myint A.S., et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): A multicentre, randomised trial. Lancet. 2009;373:811–820. doi: 10.1016/S0140-6736(09)60484-0. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

