Angiogenesis in the Avian Embryo Chorioallantoic Membrane: A Perspective on Research Trends and a Case Study on Toxicant Vascular Effects
- PMID: 33291457
- PMCID: PMC7762154
- DOI: 10.3390/jcdd7040056
Angiogenesis in the Avian Embryo Chorioallantoic Membrane: A Perspective on Research Trends and a Case Study on Toxicant Vascular Effects
Abstract
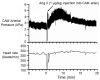
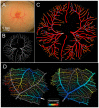
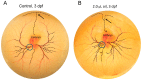
The chorioallantoic membrane (CAM) of the avian embryo is an intrinsically interesting gas exchange and osmoregulation organ. Beyond study by comparative biologists, however, the CAM vascular bed has been the focus of translational studies by cardiovascular life scientists interested in the CAM as a model for probing angiogenesis, heart development, and physiological functions. In this perspective article, we consider areas of cardiovascular research that have benefited from studies of the CAM, including the themes of investigation of the CAM's hemodynamic influence on heart and central vessel development, use of the CAM as a model vascular bed for studying angiogenesis, and the CAM as an assay tool. A case study on CAM vascularization effects of very low doses of crude oil as a toxicant is also presented that embraces some of these themes, showing the induction of subtle changes in the pattern of the CAM vasculature growth that are not readily observed by standard vascular assessment methodologies. We conclude by raising several questions in the area of CAM research, including the following: (1) Do changes in patterns of CAM growth, as opposed to absolute CAM growth, have biological significance?; (2) How does the relative amount of CAM vascularization compared to the embryo per se change during development?; and (3) Is the CAM actually representative of the mammalian systemic vascular beds that it is presumed to model?
Keywords: angiogenesis; chicken embryo; chorioallantoic membrane; crude oil.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Chorioallantoic membrane capillary bed: a useful target for studying angiogenesis and anti-angiogenesis in vivo.Anat Rec. 2001 Dec 1;264(4):317-24. doi: 10.1002/ar.10021. Anat Rec. 2001. PMID: 11745087 Review.
-
The role of fibroblast growth factor-2 in the vascularization of the chick embryo chorioallantoic membrane.J Cell Mol Med. 2002 Jul-Sep;6(3):439-46. doi: 10.1111/j.1582-4934.2002.tb00524.x. J Cell Mol Med. 2002. PMID: 12417062 Free PMC article. Review.
-
The chick embryo chorioallantoic membrane as a model system for the study of tumor angiogenesis, invasion and development of anti-angiogenic agents.Curr Cancer Drug Targets. 2005 Jun;5(4):249-66. doi: 10.2174/1568009054064624. Curr Cancer Drug Targets. 2005. PMID: 15975046 Review.
-
Utilisation of Chick Embryo Chorioallantoic Membrane as a Model Platform for Imaging-Navigated Biomedical Research.Cells. 2021 Feb 22;10(2):463. doi: 10.3390/cells10020463. Cells. 2021. PMID: 33671534 Free PMC article. Review.
-
Chicken chorioallantoic membrane angiogenesis model.Methods Mol Biol. 2012;843:47-57. doi: 10.1007/978-1-61779-523-7_5. Methods Mol Biol. 2012. PMID: 22222520
Cited by
-
Effects of neratinib on angiogenesis and the early stage of the embryo using chicken embryo as a model.Biomol Biomed. 2024 May 2;24(3):575-581. doi: 10.17305/bb.2023.9869. Biomol Biomed. 2024. PMID: 38158791 Free PMC article.
-
Multi-parametric investigations on the effects of vascular disrupting agents based on a platform of chorioallantoic membrane of chick embryos.Quant Imaging Med Surg. 2024 Feb 1;14(2):1729-1746. doi: 10.21037/qims-23-1065. Epub 2024 Jan 23. Quant Imaging Med Surg. 2024. PMID: 38415159 Free PMC article.
-
Microvascular Experimentation in the Chick Chorioallantoic Membrane as a Model for Screening Angiogenic Agents including from Gene-Modified Cells.Int J Mol Sci. 2021 Dec 31;23(1):452. doi: 10.3390/ijms23010452. Int J Mol Sci. 2021. PMID: 35008876 Free PMC article. Review.
-
In vivo evaluation of mebendazole and Ran GTPase inhibition in breast cancer model system.Nanomedicine (Lond). 2024;19(12):1087-1101. doi: 10.2217/nnm-2023-0351. Epub 2024 Apr 25. Nanomedicine (Lond). 2024. PMID: 38661720 Free PMC article.
-
In vivo label-free tissue histology through a microstructured imaging window.APL Bioeng. 2024 Jan 9;8(1):016102. doi: 10.1063/5.0165411. eCollection 2024 Mar. APL Bioeng. 2024. PMID: 38222895 Free PMC article.
References
-
- Jedelska J., Strehlow B., Bakowsky U., Aigner A., Hobel S., Bette M., Roessler M., Franke N., Teymoortash A., Werner J.A., et al. The chorioallantoic membrane assay is a promising ex vivo model system for the study of vascular anomalies. In Vivo. 2013;27:701–705. - PubMed
-
- Burggren W.W., Flores Santin J., Rojas M. Cardio-respiratory development in bird embryos: New insights from a venerable animal model. Rev. Bras. Zootec. 2016;45:709–728. doi: 10.1590/s1806-92902016001100010. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

