Characterization of AMBN I and II Isoforms and Study of Their Ca2+-Binding Properties
- PMID: 33291486
- PMCID: PMC7730623
- DOI: 10.3390/ijms21239293
Characterization of AMBN I and II Isoforms and Study of Their Ca2+-Binding Properties
Abstract
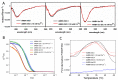
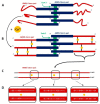
Ameloblastin (Ambn) as an intrinsically disordered protein (IDP) stands for an important role in the formation of enamel-the hardest biomineralized tissue commonly formed in vertebrates. The human ameloblastin (AMBN) is expressed in two isoforms: full-length isoform I (AMBN ISO I) and isoform II (AMBN ISO II), which is about 15 amino acid residues shorter than AMBN ISO I. The significant feature of AMBN-its oligomerization ability-is enabled due to a specific sequence encoded by exon 5 present at the N-terminal part in both known isoforms. In this study, we characterized AMBN ISO I and AMBN ISO II by biochemical and biophysical methods to determine their common features and differences. We confirmed that both AMBN ISO I and AMBN ISO II form oligomers in in vitro conditions. Due to an important role of AMBN in biomineralization, we further addressed the calcium (Ca2+)-binding properties of AMBN ISO I and ISO II. The binding properties of AMBN to Ca2+ may explain the role of AMBN in biomineralization and more generally in Ca2+ homeostasis processes.
Keywords: ameloblastin; biomineralization; calcium binding; intrinsically disordered protein (IDPs); oligomerization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Wald T., Osickova A., Sulc M., Benada O., Semeradtova A., Rezabkova L., Veverka V., Bednarova L., Maly J., Macek P., et al. Intrinsically Disordered Enamel Matrix Protein Ameloblastin Forms Ribbon-like Supramolecular Structures via an N-terminal Segment Encoded by Exon 5. J. Biol. Chem. 2013;288:22333–22345. doi: 10.1074/jbc.M113.456012. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

