Growth Hormone Upregulates Mediators of Melanoma Drug Efflux and Epithelial-to-Mesenchymal Transition In Vitro and In Vivo
- PMID: 33291663
- PMCID: PMC7761932
- DOI: 10.3390/cancers12123640
Growth Hormone Upregulates Mediators of Melanoma Drug Efflux and Epithelial-to-Mesenchymal Transition In Vitro and In Vivo
Abstract
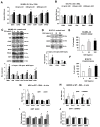
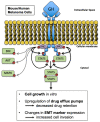
Growth hormone (GH) and the GH receptor (GHR) are expressed in a wide range of malignant tumors including melanoma. However, the effect of GH/insulin-like growth factor (IGF) on melanoma in vivo has not yet been elucidated. Here we assessed the physical and molecular effects of GH on mouse melanoma B16-F10 and human melanoma SK-MEL-30 cells in vitro. We then corroborated these observations with syngeneic B16-F10 tumors in two mouse lines with different levels of GH/IGF: bovine GH transgenic mice (bGH; high GH, high IGF-1) and GHR gene-disrupted or knockout mice (GHRKO; high GH, low IGF-1). In vitro, GH treatment enhanced mouse and human melanoma cell growth, drug retention and cell invasion. While the in vivo tumor size was unaffected in both bGH and GHRKO mouse lines, multiple drug-efflux pumps were up regulated. This intrinsic capacity of therapy resistance appears to be GH dependent. Additionally, epithelial-to-mesenchymal transition (EMT) gene transcription markers were significantly upregulated in vivo supporting our current and recent in vitro observations. These syngeneic mouse melanoma models of differential GH/IGF action can be valuable tools in screening for therapeutic options where lowering GH/IGF-1 action is important.
Keywords: epithelial-to-mesenchymal transition; growth hormone; growth hormone receptor; insulin-like growth factor-1; melanoma; multidrug efflux pumps.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Ikeno Y., Hubbard G.B., Lee S., Cortez L.A., Lew C.M., Webb C.R., Berryman D.E., List E.O., Kopchick J.J., Bartke A. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J. Gerontol. A Biol. Sci. Med. Sci. 2009;64:522–529. doi: 10.1093/gerona/glp017. - DOI - PMC - PubMed
-
- Conway-Campbell B.L., Wooh J.W., Brooks A.J., Gordon D., Brown R.J., Lichanska A.M., Chin H.S., Barton C.L., Boyle G.M., Parsons P.G., et al. Nuclear targeting of the growth hormone receptor results in dysregulation of cell proliferation and tumorigenesis. Proc. Natl. Acad. Sci. USA. 2007;104:13331–13336. doi: 10.1073/pnas.0600181104. - DOI - PMC - PubMed
-
- Van der Lely A.J., Muller A., Janssen J.A., Davis R.J., Zib K.A., Scarlett J.A., Lamberts S.W. Control of tumor size and disease activity during cotreatment with octreotide and the growth hormone receptor antagonist pegvisomant in an acromegalic patient. J. Clin. Endocrinol. Metab. 2001;86:478–481. doi: 10.1210/jcem.86.2.7206. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

